 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly of aliphatic dipeptides coupled with porphyrin and BODIPY chromophores†
Emmanouil
Nikoloudakis
 a,
Konstantina
Mitropoulou
b,
Georgios
Landrou
a,
Konstantina
Mitropoulou
b,
Georgios
Landrou
 a,
Georgios
Charalambidis
a,
Georgios
Charalambidis
 a,
Vasilis
Nikolaou
a,
Vasilis
Nikolaou
 a,
Anna
Mitraki
a,
Anna
Mitraki
 *b and
Athanassios G.
Coutsolelos
*b and
Athanassios G.
Coutsolelos
 *a
*a
aDepartment of Chemistry, University of Crete, Laboratory of Bioinorganic Chemistry, Voutes Campus, 70013 Heraklion, Crete, Greece. E-mail: acoutsol@uoc.gr
bUniversity of Crete, Department of Materials Science and Technology and Institute of Electronic Structure and Laser (I.E.S.L.), Foundation for Research and Technology – Hellas (FO.R.T.H.), Vassilika Vouton, Heraklion, 70013, Crete, Greece. E-mail: mitraki@materials.uoc.gr
First published on 2nd October 2019
Abstract
In this work, the self assembly ability of chromophores covalently linked to aliphatic dipeptides is described. Altering various parameters such as the protecting group, the solvent mixture, the dipeptide and the chromophore resulted in different nanostructures. Interestingly, a peptide–porphyrin hybrid is capable of forming a hydrogel in HFIP–water solvent mixture.
Living natural systems utilize self-assembly in order to fulfil their functions; thus, understanding the basic aspects of this process takes us a step closer to the comprehension of life.1 Numerous examples in nature have inspired supramolecular scientists to synthesize molecules with self-assembling ability aiming at the construction of materials with improved properties.2 Self-assembled architectures that mimic chlorophylls and bacteriochlorophylls in nature are of great interest for light harvesting applications.3 A variety of bioinspired building blocks have been explored for the fabrication of self-assembling molecules including peptides,4,5 nucleic acids,6,7 peptide nucleic acids,8,9 lipids etc. Of the small peptides, diphenylalanine (FF), the basic structural motif for the Alzheimer's beta amyloid polypeptide, is an aromatic dipeptide that has been extensively investigated for its self-assembling properties.10,11 More specifically, a great number of chromophores such as porphyrins,12–15 boron-dipyrromethenes,16 corroles,17 polyoxometallates18 and ferrocene19 have been covalently connected to FF resulting in hybrids that retained the ability to form well-defined nanostructures. In addition, small aliphatic peptides have also been investigated for their self-assembly ability.20 In a recent example, Yan and co-workers reported that a short peptide based on Isoleucine was employed to induce the self-assembly to hydrophobic porphyrins, which were used as photosensitizers in two-photon PDT.21 Light harvesting applications for self-assembled porphyrins have also been reported, such as photocatalytic hydrogen generation in the presence of platinum nanoparticles.22 Moreover, the self-assembling ability of short peptides can lead to the formation of hydrogels, which can improve the properties of chromophores.23 Hydrogels that contain porphyrin chromophores can be used for hydrogen production,24 water oxidation,25 photodynamic therapy,26 fluorescence-guided monitoring27 and photocurrent generation.28 However, in the above hydrogel systems, the chromophore was not covalently attached to the peptide gelator molecule.
In this communication, we report the self-assembly ability of porphyrin and BODIPY hybrids that are covalently coupled to aliphatic dipeptides bearing various protecting groups (Scheme 1). Namely, Ile-Ile and Ala-Ile dipeptides with Boc-, Fmoc-, Z-, and -OMe protecting groups were investigated using the “good–bad” solvent self-assembling protocol, leading to distinctive supramolecular architectures. One of the hybrids, Fmoc-Ile-Ile-TPP formed a hydrogel in HFIP–water solvent mixture. To the best of our knowledge, this is the first example of hydrogel formation by a single hybrid dipeptide-porphyrin molecule and this opens new avenues for possible applications of these self-assembled hybrids.
 | ||
| Scheme 1 Structures of Boc-Ala-Ile-TPP, Z-Ala-Ile-TPP, Boc-Ile-Ile-TPP, TPP-Ile-Ile-OMe, Fmoc-Ala-Ile-TPP, Fmoc-Ile-Ile-TPP, Boc-Ala-Ile-BDP and Boc-Ile-Ile-BDP. | ||
The synthetic approach for the preparation of Boc-Ala-Ile-TPP and Boc-Ile-Ile-TPP is illustrated in Scheme S1 (ESI†). The first step is the N-protection of the commercially available dipeptides (Ile-Ile-OH and Ala-Ile-OH) with Boc2O in the presence of NaHCO3, followed by the covalent connection with TPP-NH2 using DCC/HOBt amide coupling reagents. The synthesis of TPP-Ile-Ile-OMe begins with the methyl ester-protection of the C-terminus of Ile-Ile-OH dipeptide with SOCl2 and MeOH followed by an amide coupling reaction with TPP-COOH. In order to successfully prepare the Fmoc protected hybrids, it is necessary to “construct” the dipeptide onto the porphyrin macrocycle starting with an amide coupling reaction between Fmoc-Ile-COOH and TPP-NH2. Subsequently, we removed the Fmoc protection group using piperidine in DMF yielding the NH2-Ile-TPP hybrid. The final step is the covalent connection of the hybrid via amide coupling to the Fmoc-Ala-COOH or Fmoc-Ile-COOH resulting in Fmoc-Ala-Ile-TPP or Fmoc-Ile-Ile-TPP, respectively (Scheme S2, ESI†). In addition, the Boc-protected dipeptides were also coupled with boron-dipyrromethene chromophores following an experimental approach similar to the preparation of the corresponding porphyrin hybrids (Scheme S3, ESI†). All the final and intermediate compounds were fully characterized with 1H and 13C NMR spectroscopies (Fig. S1–S16, ESI†).
The self-assembly behavior of the synthesized bio-conjugates was examined through Scanning Electron Microscopy (SEM). For the preparation of the samples, the corresponding compounds were dissolved in a chaotropic “good” solvent, namely 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), and subsequently a “bad” solvent was introduced such as ethanol (EtOH), methanol (MeOH) or water (H2O). Noteworthily, the “bad” solvents were utilized in order to induce the self-assembly process. We investigated the effect of various parameters on the formation of the self-assembly, such as the protecting group, the nature of the dipeptide, the solvent mixture as well as the ratio of the “good”/“bad” solvent.
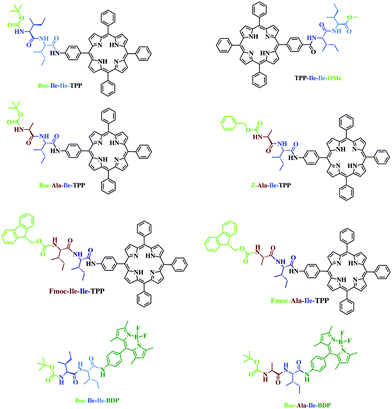
As it is illustrated in Fig. 1a, theBoc-Ile-Ile-TPP hybrid assembled into uniform spiky spheres by using the HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 solvent system at 1 mM final concentration. The supramolecular architectures were formed within an hour after the addition of the bad solvent and did not evolve either for the next 24 h or the following week, proving the inherent stability of the system (Fig. S17, ESI†). By simply changing the “bad” solvent from MeOH to EtOH different morphologies on the self- assembled structures were obtained. To be more specific, Boc-Ile-Ile-TPP gave rise to flake – shape structures in HFIP–EtOH 2
8 solvent system at 1 mM final concentration. The supramolecular architectures were formed within an hour after the addition of the bad solvent and did not evolve either for the next 24 h or the following week, proving the inherent stability of the system (Fig. S17, ESI†). By simply changing the “bad” solvent from MeOH to EtOH different morphologies on the self- assembled structures were obtained. To be more specific, Boc-Ile-Ile-TPP gave rise to flake – shape structures in HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 mixed solvent system at 1 mM final concentration after 24 hours (Fig. 1b). Notably, the flakes were not observed after one hour, and thus their formation needs more time than the spiky spheres, but remained stable also after one week (Fig. S18, ESI†). Well-dispersed spherical assemblies with uniform size were observed by using water as a “bad” solvent. Interestingly, the size of these spheres was larger than the spheres obtained with MeOH, but lacked the spiky surface (Fig. S19, ESI†).
8 mixed solvent system at 1 mM final concentration after 24 hours (Fig. 1b). Notably, the flakes were not observed after one hour, and thus their formation needs more time than the spiky spheres, but remained stable also after one week (Fig. S18, ESI†). Well-dispersed spherical assemblies with uniform size were observed by using water as a “bad” solvent. Interestingly, the size of these spheres was larger than the spheres obtained with MeOH, but lacked the spiky surface (Fig. S19, ESI†).
 | ||
Fig. 1 Self-assembly behavior of (a) Boc-Ile-Ile-TPP in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (b) Boc-Ile-Ile-TPP in HFIP–EtOH 2 8, (b) Boc-Ile-Ile-TPP in HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (c) Boc-Ala-Ile-TPP in HFIP–MeOH 2 8, (c) Boc-Ala-Ile-TPP in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and (d) Boc-Ala-Ile-TPP in HFIP–EtOH 2 8 and (d) Boc-Ala-Ile-TPP in HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. 8. | ||
We replaced one of the two isoleucine amino acids with an alanine and the Boc-Ala-Ile-TPP hybrid assembled into uniform spiky spheres by using HFIP–MeOH and HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 solvent systems (Fig. 1c and d) while the use of water did not result in defined self-assemblies (Fig. S20, ESI†). The replacement of Boc- protecting group with the Z-(benzyl carbamate) Z-Ala-Ile-TPP showed also uniform spheres with spiky surfaces in the same examined conditions (Fig. 2 and Fig. S21, ESI†). The influence of another aromatic protecting group, Fmoc-, was investigated as well. The Fmoc-Ala-Ile-TPP hybrid (Fig. 2 and Fig. S22, ESI†) was able to form spherical architectures in HFIP–MeOH and HFIP–EtOH 2
8 solvent systems (Fig. 1c and d) while the use of water did not result in defined self-assemblies (Fig. S20, ESI†). The replacement of Boc- protecting group with the Z-(benzyl carbamate) Z-Ala-Ile-TPP showed also uniform spheres with spiky surfaces in the same examined conditions (Fig. 2 and Fig. S21, ESI†). The influence of another aromatic protecting group, Fmoc-, was investigated as well. The Fmoc-Ala-Ile-TPP hybrid (Fig. 2 and Fig. S22, ESI†) was able to form spherical architectures in HFIP–MeOH and HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 solvent conditions. In the above cases of Ala-Ile dipeptide hybrids, no flakes were obtained and water was not an appropriate solvent for the formation of self-assembled nanostructures.
8 solvent conditions. In the above cases of Ala-Ile dipeptide hybrids, no flakes were obtained and water was not an appropriate solvent for the formation of self-assembled nanostructures.
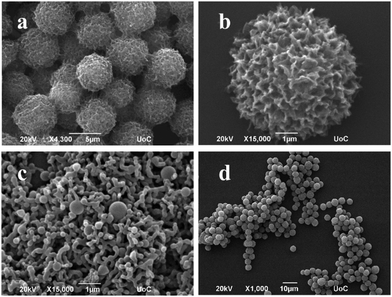 | ||
Fig. 2 Self-assembly behavior of (a) Z-Ala-Ile-TPP in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (b) Z-Ala-Ile-TPP in HFIP–EtOH 2 8, (b) Z-Ala-Ile-TPP in HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (c) Fmoc-Ala-Ile-TPP in HFIP–MeOH 2 8, (c) Fmoc-Ala-Ile-TPP in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and (d) Fmoc-Ala-Ile-TPP in HFIP–EtOH 2 8 and (d) Fmoc-Ala-Ile-TPP in HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. 8. | ||
A completely different supramolecular architecture was observed in the case of the Fmoc-Ile-Ile-TPP hybrid (Fig. 3a, b and Fig. S23, ESI†). It was able to form spiky nano-assemblies, which is not common in these kinds of systems, after dissolving in HFIP and using MeOH or EtOH as a bad solvent. Surprisingly, by using water as a “bad” solvent, hydrogels were formed (Fig. 4a, b and Fig. S24, ESI†). Hydrogels consist of an interconnected fibrillar network with high water content (Fig. 4b) and can be used in many biomedical applications. This was an unexpected result since, as far as we know, it is the first chromophore molecule that forms a hydrogel in the absence of any other gelator molecules. Moreover, we examined the effect of protecting the carboxyl terminus of the dipeptide, as far as it may influence the self-assembly process. More specifically, the TPP-Ile-Ile-OMe hybrid was studied in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and HFIP–EtOH 2
8 and HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and formed flakes by applying MeOH or spheres when EtOH was used as a “bad” solvent (Fig. 3c and d). The above results provide real evidence that the choice of the protecting group, the nature of the dipeptide and the solvent mixture can lead to distinctive supramolecular architectures. One can conclude that the Ile-Ile dipeptide is able to induce more distinctive architectures, namely spheres, flakes and spikes, while Ala-Ile can lead only to spherical assemblies.
8 and formed flakes by applying MeOH or spheres when EtOH was used as a “bad” solvent (Fig. 3c and d). The above results provide real evidence that the choice of the protecting group, the nature of the dipeptide and the solvent mixture can lead to distinctive supramolecular architectures. One can conclude that the Ile-Ile dipeptide is able to induce more distinctive architectures, namely spheres, flakes and spikes, while Ala-Ile can lead only to spherical assemblies.
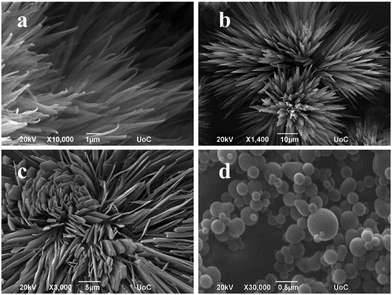 | ||
Fig. 3 Self-assembly behavior of (a) Fmoc-Ile-Ile-TPP in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and (b) Fmoc-Ile-Ile-TPP in HFIP–EtOH 2 8 and (b) Fmoc-Ile-Ile-TPP in HFIP–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (c) TPP-Ile-Ile-OMe in HFIP–MeOH 2 8, (c) TPP-Ile-Ile-OMe in HFIP–MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and (d) TPP-Ile-Ile-OMe in HFIP–H2O 2 8 and (d) TPP-Ile-Ile-OMe in HFIP–H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. 8. | ||
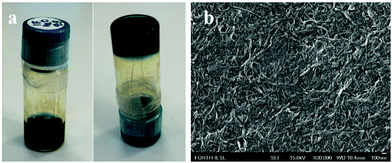 | ||
Fig. 4 (a) Photographs of the hydrogel formed from the Fmoc-Ile-Ile-TPP hybrid in HFIP–H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 solvent mixture at 1 mM final concentration. (b) FESEM image of the formed hydrogel. 8 solvent mixture at 1 mM final concentration. (b) FESEM image of the formed hydrogel. | ||
The self-assembling behavior of another chromophore, namely boron-dipyrromethene, was also investigated in this work. Therefore we synthesized hybrids Boc-Ala-Ile-BDP and Boc-Ile-Ile-BDP and tested their behavior in the same solvent systems as the corresponding porphyrin hybrids. Interestingly, no self-assembling ability was observed in HFIP–EtOH and HFIP–MeOH mixtures (Fig. S25, ESI†). This can be attributed to the partial solubility of BODIPY molecules in ethanol and methanol. Since BDP-dipeptide hybrids are quite soluble in EtOH and MeOH, there is not an actual “bad” solvent to induce self-assembly in these two systems. On the other hand, when water was used as a “bad” solvent, well defined spherical nanostructures with a uniform size were formed (Fig. 5).
 | ||
Fig. 5 Self-assembly behavior of (a) Boc-Ala-Ile-BDP in HFIP–H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, and (b) Boc-Ile-Ile-BDP in HFIP–H2O 2 8, and (b) Boc-Ile-Ile-BDP in HFIP–H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. 8. | ||
The synthesized hybrids were studied with UV-vis absorption spectroscopy in solution and in the solid state. The broadening and red shifts of all bands of the chromophore (and especially the Soret band) observed in all porphyrin hybrids in relation to the respective bands of the compounds in solution (DCM) indicate the formation of J-aggregates (side-by-side) of the porphyrin moiety in the assemblies (Fig. S26–S29 and Table S1, ESI†). Equivalent observations were made during the fluorescence studies, where the observed red shift supports the formation of J-aggregates (Fig. S30, S31 and Table S2, ESI†).
In conclusion, this work describes the synthesis and characterization of porphyrin and BODIPY hybrids coupled with aliphatic peptides that are able to self-assemble in mixed solvent systems. More specifically, two dipeptides namely Ile-Ile and Ile-Ala bearing a series of protecting groups (Fmoc-, Boc-, -Z, -OCH3) were connected with porphyrin and BODIPY chromophores. To the best of our knowledge, this is the first example where Ile-Ile and Ala-Ile dipeptides are covalently attached to chromophores in order to transfer their self-assembling properties. The resulting conjugates were able to form spherical and spiky nanostructures depending on the protecting group, the solvent mixture and the nature of the dipeptide and the chromophore. Moreover, the Fmoc-Ile-Ile-TPP hybrid was able to form hydrogels in HFIP–H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 solvent mixture at 1 mM final concentration. The BODIPY hybrids were not able to self-assemble when MeOH and EtOH were used as “bad” solvents, probably due to their limited solubility in these solvents, however they assembled into uniform nanometric spheres in the HFIP–H2O solvent system. The various self-assembled configurations that can be adopted depending on the conditions described above leave “plenty of room” for a variety of applications. In particular, rough and “spiky” spheres could be used as catalysts due to their larger surface.29 Moreover, “smooth spheres” with nanometric dimensions can easily enter cells and be applied in drug delivery and photodynamic therapy.30 Finally, hydrogels can be utilized for biomedical applications.31
8 solvent mixture at 1 mM final concentration. The BODIPY hybrids were not able to self-assemble when MeOH and EtOH were used as “bad” solvents, probably due to their limited solubility in these solvents, however they assembled into uniform nanometric spheres in the HFIP–H2O solvent system. The various self-assembled configurations that can be adopted depending on the conditions described above leave “plenty of room” for a variety of applications. In particular, rough and “spiky” spheres could be used as catalysts due to their larger surface.29 Moreover, “smooth spheres” with nanometric dimensions can easily enter cells and be applied in drug delivery and photodynamic therapy.30 Finally, hydrogels can be utilized for biomedical applications.31
This research was funded by the General Secretariat for Research and Technology (GSRT) and Hellenic Foundation for Research and Innovation (HFRI) (project code: 508). This research has also been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH – CREATE – INNOVATE (project code: T1EDK-01504). In addition, this research has been co-financed by the European Union and Greek national funds through the Regional Operational Program “Crete 2014–2020”, project code OPS: 5029187. Moreover, the European Commission's Seventh Framework Program (FP7/2007-2013) under grant agreement no. 229927 (FP7-REGPOT-2008-1, Project BIO-SOLENUTI) and the Special Research Account of the University of Crete are gratefully acknowledged for the financial support of this research.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- T. S. Balaban, A. D. Bhise, M. Fischer, M. Linke-Schaetzel, C. Roussel and N. Vanthuyne, Angew. Chem., Int. Ed., 2003, 42, 2140–2144 CrossRef CAS PubMed.
- K. J. Channon, G. L. Devlin and C. E. MacPhee, J. Am. Chem. Soc., 2009, 131, 12520–12521 CrossRef CAS PubMed.
- K. Tao, B. Xue, S. Frere, I. Slutsky, Y. Cao, W. Wang and E. Gazit, Chem. Mater., 2017, 29, 4454–4460 CrossRef CAS PubMed.
- K. Tao, P. Makam, R. Aizen and E. Gazit, Science, 2017, 358, eaam9756 CrossRef PubMed.
- M. V. Ishutkina, A. R. Berry, R. Hussain, O. G. Khelevina, G. Siligardi and E. Stulz, Eur. J. Org. Chem., 2018, 5054–5059 CrossRef CAS PubMed.
- K. Wang, M. You, Y. Chen, D. Han, Z. Zhu, J. Huang, K. Williams, C. J. Yang and W. Tan, Angew. Chem., Int. Ed., 2011, 50, 6098–6101 CrossRef CAS PubMed.
- E. Nikoloudakis, K. Karikis, J. Han, C. Kokotidou, A. Charisiadis, F. Folias, A. M. Douvas, A. Mitraki, G. Charalambidis, X. Yan and A. G. Coutsolelos, Nanoscale, 2019, 11, 3557–3566 RSC.
- O. Berger, E. Yoskovitz, L. Adler-Abramovich and E. Gazit, Adv. Mater., 2016, 28, 2195–2200 CrossRef CAS PubMed.
- M. Reches and E. Gazit, Science, 2003, 300, 625–627 CrossRef CAS PubMed.
- N. Kol, L. Adler-Abramovich, D. Barlam, R. Z. Shneck, E. Gazit and I. Rousso, Nano Lett., 2005, 5, 1343–1346 CrossRef CAS PubMed.
- G. Charalambidis, E. Kasotakis, T. Lazarides, A. Mitraki and A. G. Coutsolelos, Chem. – Eur. J., 2011, 17, 7213–7219 CrossRef CAS PubMed.
- G. Charalambidis, E. Georgilis, M. K. Panda, C. E. Anson, A. K. Powell, S. Doyle, D. Moss, T. Jochum, P. N. Horton, S. J. Coles, M. Linares, D. Beljonne, J. V. Naubron, J. Conradt, H. Kalt, A. Mitraki, A. G. Coutsolelos and T. S. Balaban, Nat. Commun., 2016, 7, 12657 CrossRef CAS PubMed.
- G. Charalambidis, K. Karikis, E. Georgilis, B. L. M'Sabah, Y. Pellegrin, A. Planchat, B. Lucas, A. Mitraki, J. Boucle, F. Odobel and A. G. Coutsolelos, Sustainable Energy Fuels, 2017, 1, 387–395 RSC.
- W. Nuansing, E. Georgilis, T. V. A. G. de Oliveira, G. Charalambidis, A. Eleta, A. G. Coutsolelos, A. Mitraki and A. M. Bittner, Part. Part. Syst. Charact., 2014, 31, 88–93 CrossRef CAS.
- K. Karikis, A. Butkiewicz, F. Folias, G. Charalambidis, C. Kokotidou, A. Charisiadis, V. Nikolaou, E. Nikoloudakis, J. Frelek, A. Mitraki and A. G. Coutsolelos, Nanoscale, 2018, 10, 1735–1741 RSC.
- K. Karikis, E. Georgilis, G. Charalambidis, A. Petrou, O. Vakuliuk, T. Chatziioannou, I. Raptaki, S. Tsovola, I. Papakyriacou, A. Mitraki, D. T. Gryko and A. G. Coutsolelos, Chem. – Eur. J., 2016, 22, 11245–11252 CrossRef CAS PubMed.
- E. Nikoloudakis, K. Karikis, M. Laurans, C. Kokotidou, A. Sole-Daura, J. J. Carbo, A. Charisiadis, G. Charalambidis, G. Izzet, A. Mitraki, A. M. Douvas, J. M. Poblet, A. Proust and A. G. Coutsolelos, Dalton Trans., 2018, 47, 6304–6313 RSC.
- Z. Sun, Z. Li, Y. He, R. Shen, L. Deng, M. Yang, Y. Liang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 13379–13386 CrossRef CAS PubMed.
- C. H. Gorbitz, Chemistry, 2007, 13, 1022–1031 CrossRef PubMed.
- J. Li, A. Wang, P. Ren, X. Yan and S. Bai, Chem. Commun., 2019, 55, 3191–3194 RSC.
- Y. Liu, L. Wang, H. Feng, X. Ren, J. Ji, F. Bai and H. Fan, Nano Lett., 2019, 19, 2614–2619 CrossRef CAS PubMed.
- Y. Xia, B. Xue, M. Qin, Y. Cao, Y. Li and W. Wang, Sci. Rep., 2017, 7, 9691 CrossRef PubMed.
- P. Liao, Y. Hu, Z. Liang, J. Zhang, H. Yang, L.-Q. He, Y.-X. Tong, J.-M. Liu, L. Chen and C.-Y. Su, J. Mater. Chem. A, 2018, 6, 3195–3201 RSC.
- J. H. Kim, D. H. Nam, Y. W. Lee, Y. S. Nam and C. B. Park, Small, 2014, 10, 1272–1277 CrossRef CAS.
- J. A. Gonzalez-Delgado, P. M. Castro, A. Machado, F. Araujo, F. Rodrigues, B. Korsak, M. Ferreira, J. P. Tome and B. Sarmento, Int. J. Pharm., 2016, 510, 221–231 CrossRef CAS PubMed.
- J. F. Lovell, A. Roxin, K. K. Ng, Q. Qi, J. D. McMullen, R. S. DaCosta and G. Zheng, Biomacromolecules, 2011, 12, 3115–3118 CrossRef CAS PubMed.
- L. Feng, A. Wang, P. Ren, M. Wang, Q. Dong, J. Li and S. Bai, Colloid Interface Sci. Commun., 2018, 23, 29–33 CrossRef CAS.
- T. Fuchigami and K.-i. Kakimoto, J. Mater. Res., 2017, 32, 3326–3332 CrossRef CAS.
- Q. Zou, M. Abbas, L. Zhao, S. Li, G. Shen and X. Yan, J. Am. Chem. Soc., 2017, 139, 1921–1927 CrossRef CAS PubMed.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc06125h |
| This journal is © The Royal Society of Chemistry 2019 |
