Long-term stabilized high-density CuBi2O4/NiO heterostructure thin film photocathode grown by pulsed laser deposition†
Abstract
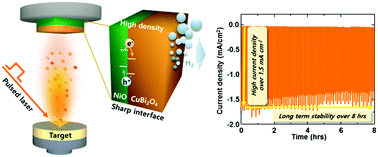
Harvesting sustainable hydrogen through water-splitting requires a durable photoelectrode to achieve high efficiency and long lifetime. Dense, uniform CuBi2O4/NiO thin film photocathodes grown by pulsed laser deposition achieved photocurrent density over 1.5 mA cm−2 at 0.4 VRHE and long-term chronoamperometric stability for over 8 hours.


 Please wait while we load your content...
Please wait while we load your content...