Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intercepted dehomologation of aldoses by N-heterocyclic carbene catalysis – a novel transformation in carbohydrate chemistry†
Markus
Draskovits
 ,
Hubert
Kalaus
,
Hubert
Kalaus
 ,
Christian
Stanetty
,
Christian
Stanetty
 * and
Marko D.
Mihovilovic
* and
Marko D.
Mihovilovic

Institute of Applied Synthetic Chemistry, TU Wien, 1060 Vienna, Austria. E-mail: christian.stanetty@tuwien.ac.at; Tel: +43-(1)58801-163619
First published on 19th September 2019
Abstract
The development of an N-heterocyclic carbene (NHC) catalysed intercepted dehomologation of aldoses is reported. The unique selectivity of NHCs for aldehydes is exploited in the complex context of reducing sugars. Examples of strong substrate governance for either intercepted dehomologation or a subsequent redox-lactonisation were identified and mechanistically understood. More importantly, it was shown that catalyst design allowed the tuning of the selectivity of the reaction with structurally unbiased starting materials towards either of the two scenarios.
Carbohydrates are the most abundant class of all biomolecules formed in Nature and are found in all living organisms. They serve as structural components of cells, as energy sources within metabolic cycles and also play a major role in the recognition process of biomolecules.1 Despite their ubiquitous nature, only a limited number of representatives of the family of carbohydrates are readily available, requiring great synthetic efforts to provide the full range of carbohydrates.2 Great effort and progress have been made in the assembly of complex oligosaccharides, often based on elaborative protecting group chemistry to overcome the challenges of both regio- and stereoselectivity with respect to the hydroxyl functionalities.3 Intriguingly, the intrinsically reactive part of an aldose, the aldehyde moiety, is targeted far less in classic carbohydrate chemistry.4 The established methods include addition of organometallics (Mg, In, and Zn)5 or carbanions (Kiliani–Fischer)6 to achieve an elongation. Alternatively, also dehomologation towards shortened carbohydrates (e.g. by the Wohl degradation) initiate by addressing the aldehyde moiety.7 Requirements of stoichiometric amounts of reagents and/or limited compatibility with unprotected aldoses due to solubility and cross-reactivity with the free hydroxyl groups are major challenges in this field.8
In this light, we perceived great potential for transformations triggered by the highly aldehyde-specific interaction of N-heterocyclic carbenes (NHCs) with the (anomeric) carbonyl moiety which we have just started to exploit. After the isolation of the first bench-stable carbene by Arduengo,9 the number of applications of NHCs has vastly increased over the last decades. The main areas of research have been their utilisation as ligands in transition-metal catalysed reactions10 and as organocatalysts.11 The field of organocatalysis is dominated by processes rooted in the umpolung of aldehydes,12 triggering benzoin or Stetter type reactions13 as well as more sophisticated follow-up transformations.14
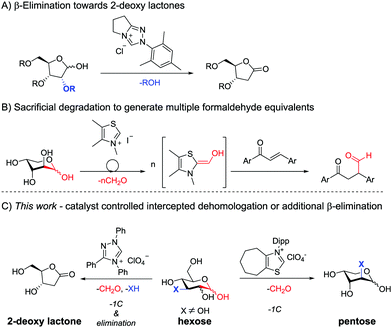
The applications of NHCs within the realm of reducing sugars, as aldehyde species, are extremely scarce (see Scheme 1).15 Wendeborn et al. attempted to perform Stetter reactions of fully protected reducing aldoses but instead observed dominant β-elimination towards protected 2-deoxy lactones,16 consistent with earlier studies on α-reducible aldehydes.17 The group of Chi achieved the catalytic activation of fully unprotected aldoses by NHCs generating multiple nucleophilic formaldehyde equivalents via a retro-benzoin reaction in an uncontrolled fashion. The thereby achieved formylation of α,β-unsaturated compounds in a subsequent Stetter reaction was the goal of the study, with the sugars being utilised as a sacrificial feedstock.18
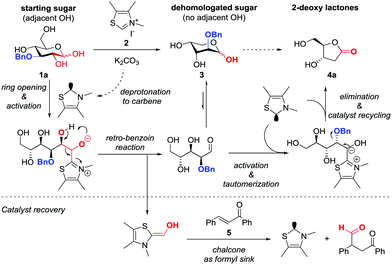
Inspired by these works and building upon the proposed mechanism, we set out to develop an NHC controlled intercepted dehomologation methodology of carbohydrate derivatives. See Scheme 2 for an outline of the mechanistic consideration with 3-O-Bn-glucose 1a as an example. First, the carbene, formed in situ from corresponding 2, attacks the aldehyde of the open-chain form of the carbohydrate, which is in equilibrium with the typically dominant lactol form.19 In the presence of an adjacent OH group a retro-benzoin reaction delivers the shortened carbohydrate 2-O-Bn arabinose 3. The concomitantly formed Breslow-intermediate of formaldehyde undergoes a Stetter reaction with a chalcone 5, thereby regenerating the catalyst (Scheme 2, bottom). In the case of unprotected sugars the first cycle is reiterated,18 which cannot occur in the absence of an adjacent OH-group. Nonetheless, if the intercepting group can act as a leaving group, elimination of e.g. BnOH from the Breslow intermediate occurs, upon activation of the carbonyl of 3. Upon the tautomerisation of the resultant acylazolium species and displacement of the NHC by ring closure deoxy lactone 4a can be formed.16
In our study, we were first of all aiming to deliver a principle proof of concept of the above approach and further investigate which structural features of both substrate and/or catalyst are required to allow for high selectivity for sole dehomologation or additional elimination. We initially evaluated several suitably semi-protected reducing sugars with the thiazolium pre-catalyst 2 under general conditions.18 Compounds 6 and 8 gave the cleanest conversions and reasonable isolated yields delivering the desired proof of concept for successfully intercepted dehomologation (1 and 2 carbons, respectively). However, the isolated products turned out to be representatives of two borderline scenarios (Scheme 3, top and middle). Arabinose acetonide 620 furnished the desired dehomologated product erythrose acetonide 7a as the main product (isolated as acetate 7b). In contrast, treatment of benzylidene glucose 8 led to predominant formation of 2-hydroxy-γ-butyrolactone 9a, thus reflecting successful double dehomologation but with concomitant redox-lactonisation via elimination of benzaldehyde. According to our hypothesis the stability of the starting material, intermediates and final products as lactols is responsible for the observed differences in selectivity. Consequently, 3-O-Bn glucose 1a gave mixtures of the dehomologated products 2-O-Bn arabinose 3 and 2-deoxy-ribonolactone 4a upon NHC-catalysed elimination of BnOH (Scheme 3, bottom) reflecting similar lactol stabilities of 1a/3. To corroborate the above mechanism, we subjected 2-O-Bn arabinose 3 to the standard reaction conditions and confirmed deoxy lactone 4a as the main product. Furthermore, by installing a better leaving group at O3 as in 3-O-(p-nitrophenyl) glucose 10 the subsequent elimination to 4a was dominant under the same reaction conditions (see the ESI†).
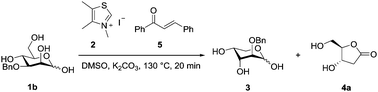
Our ultimate goal was to achieve catalyst control over the selectivity between intercepted dehomologation and subsequent redox-lactonisation. Therefore, we selected 3-O-Bn glucose 1a and its 2O-epimer 3-O-Bn mannose 1b as model compounds for the re-evaluation of the reaction conditions18 and further catalyst development. In advance, they serve as probes for potential influence of the relative stereochemistry at C2/C3. Both substrates and their common reaction products (3 and 4a) are accessible in a few chemical steps (see the ESI†). A reliable procedure for an efficient and quantitative screening was required, given the complexity of crude reaction mixtures. We developed a method based on a solid phase extraction (SPE) with subsequent derivatisation of all carbohydrate species to allow quantification via a calibrated GC protocol (see details in the ESI†).21
Preliminary studies clearly showed that sub-stoichiometric amounts of base compared to the pre-catalyst (0.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 equiv.) and a sufficient reservoir of chalcone (2.0 equiv.) are mandatory requirements. Under these conditions, the influence of solvent, type of base as well as lower and higher temperatures has been evaluated which is summarized for 1b as a starting material in Table 1 (and in the ESI† for 1a which performed similarly).18 The highest recovery of identified products was generally observed in DMSO (Table 1, entries 1–4) under conventional heating. The second generally applicable solvent was CH3CN which was preferred for preparative experiments under μW-heating (vide infra). The use of stock solutions in DMSO led to increased reproducibility and facilitated the overall work flow. Aiming to replace insoluble K2CO3, Li2CO3 and DBU were assessed as bases (Table 1, entries 5 and 6), however, the reaction did not occur in the case of DBU and led to a decrease in conversion with Li2CO3. Generally, time courses were conducted to make sure that the reported data points were representative ones (see the ESI†). A significant decrease of identified products with prolonged reaction time was observed, indicating competing not yet understood side-reactions. Experiments at higher or lower temperatures (Table 1, entries 7–9) did not give any improvements over the standard conditions. With the optimized reaction conditions, we turned our attention towards the catalysts, a usually decisive element in many previous NHC-studies.22
0.25 equiv.) and a sufficient reservoir of chalcone (2.0 equiv.) are mandatory requirements. Under these conditions, the influence of solvent, type of base as well as lower and higher temperatures has been evaluated which is summarized for 1b as a starting material in Table 1 (and in the ESI† for 1a which performed similarly).18 The highest recovery of identified products was generally observed in DMSO (Table 1, entries 1–4) under conventional heating. The second generally applicable solvent was CH3CN which was preferred for preparative experiments under μW-heating (vide infra). The use of stock solutions in DMSO led to increased reproducibility and facilitated the overall work flow. Aiming to replace insoluble K2CO3, Li2CO3 and DBU were assessed as bases (Table 1, entries 5 and 6), however, the reaction did not occur in the case of DBU and led to a decrease in conversion with Li2CO3. Generally, time courses were conducted to make sure that the reported data points were representative ones (see the ESI†). A significant decrease of identified products with prolonged reaction time was observed, indicating competing not yet understood side-reactions. Experiments at higher or lower temperatures (Table 1, entries 7–9) did not give any improvements over the standard conditions. With the optimized reaction conditions, we turned our attention towards the catalysts, a usually decisive element in many previous NHC-studies.22
| Entry | Deviation from standard conditions | 1b [%] | 3a [%] | 4a [%] | Sumb [%] |
|---|---|---|---|---|---|
a Reaction conditions: 1b/2/base/5 = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.20 0.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 (molar ratio), 20 min.
b Based on calibrated GC.
c Reactions performed under μW irradiation. 2.00 (molar ratio), 20 min.
b Based on calibrated GC.
c Reactions performed under μW irradiation.
|
|||||
| 1 | None | 6 | 48 | 43 | 96 |
| 2 | Solvent:MeCNc | 0 | 4 | 63 | 67 |
| 3 | Solvent:DMFc | 0 | 9 | 36 | 45 |
| 4 | Solvent:EtOHc | 0 | 3 | 30 | 33 |
| 5 | Base:Li2CO3 | 14 | 46 | 18 | 78 |
| 6 | Base:DBU | 83 | 14 | 4 | 101 |
| 7 | T = 90 °C/320 min | 7 | 18 | 42 | 67 |
| 8 | T = 110 °C/80 min | 0 | 4 | 44 | 48 |
| 9 | T = 150 °C/10 min | 0 | 2 | 18 | 20 |
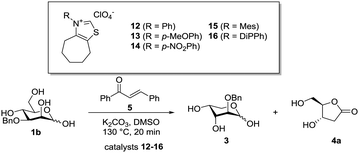
We exchanged the N-alkyl thiazolium salt 2 of a family of N-aryl-thiazolium precatalysts based on Glorius’ cycloheptyl scaffold (Table 2 and ESI,† for synthesis of the catalysts) to allow for both steric and electronic tuning of the catalyst.23 Already, phenyl-substituted thiazolium salt 12 gave an increased ratio of 2-O-Bn arabinose 3 over lactone 4a, although with a slower conversion. Formal substitution of the phenyl substituent with a p-methoxy group (13) resulted in an increased reactivity (total conversion) without a beneficial effect on the selectivity. Introduction of an electron withdrawing substituent p-nitrophenyl (14) led to no reaction, which is in good agreement with the decreased nucleophilicity of the carbene (Table 2, entries 3 and 4). With increased steric bulk of the aromatic substituent (Table 2, entries 5 and 6) the selectivity and conversion improved significantly. The mesityl substituent (15) gave a 3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the dehomologated to the elimination product, and with diisopropylphenyl substituted thiazolium salt 16, a 6
1 ratio of the dehomologated to the elimination product, and with diisopropylphenyl substituted thiazolium salt 16, a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
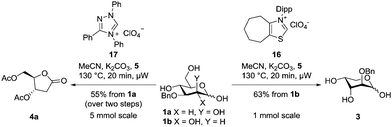
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in favour of the dehomologated product 2-O-Bn arabinose 3 was observed. In a separate experiment 3 was reacted with precatalyst 16 (Table 2, entry 7) giving no notable conversion to deoxy lactone 4a, confirming a strong selectivity of 16 for the reaction with 1b over 3. The GC-data obtained in DMSO were validated by a preparative experiment in MeCN and under microwave irradiation, which led to a comparable isolated yield (1 mmol scale, Scheme 4). The fact that the gluco-analogue 1a gave inferior results (ESI†) for us strongly indicates competing side reactions which dominate in the case of species of high lactol stability (low open chain content).24 The above data was initially found to show that increasing the steric congestion around the carbene centre increases the selectivity for the intercepted dehomologation. We assumed this to be due to the steric clash between the 2-O-substituent of 3 and the diisopropyl substituent of the catalyst.
1 ratio in favour of the dehomologated product 2-O-Bn arabinose 3 was observed. In a separate experiment 3 was reacted with precatalyst 16 (Table 2, entry 7) giving no notable conversion to deoxy lactone 4a, confirming a strong selectivity of 16 for the reaction with 1b over 3. The GC-data obtained in DMSO were validated by a preparative experiment in MeCN and under microwave irradiation, which led to a comparable isolated yield (1 mmol scale, Scheme 4). The fact that the gluco-analogue 1a gave inferior results (ESI†) for us strongly indicates competing side reactions which dominate in the case of species of high lactol stability (low open chain content).24 The above data was initially found to show that increasing the steric congestion around the carbene centre increases the selectivity for the intercepted dehomologation. We assumed this to be due to the steric clash between the 2-O-substituent of 3 and the diisopropyl substituent of the catalyst.
| Entry | Starting material | Catalyst | 1b [%] | 3 [%] | 4a [%] | Ratio 3/4a |
|---|---|---|---|---|---|---|
a Reaction conditions: 1b/precatalyst/K2CO3/5 = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.20 0.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 (molar ratio), 20 min.
b Based on calibrated GC.
c
3 used instead of 1b. 2.00 (molar ratio), 20 min.
b Based on calibrated GC.
c
3 used instead of 1b.
|
||||||
| 1 | 1b | 2 | 6 | 48 | 43 | 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 1b | 12 | 63 | 29 | 9 | 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 1b | 13 | 43 | 30 | 17 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 1b | 14 | 93 | 2 | 4 | — |
| 5 | 1b | 15 | 25 | 39 | 10 | 3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 1b | 16 | 7 | 78 | 13 | 6.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7c | 3 | 16 | — | 82 | 6 | — |
Therefore, we next evaluated triphenyl substituted triazolium salts as precatalysts, initially aiming at introducing additional steric bulk around the carbene centre. We discovered that the parent Enders’ triphenyl triazolium salt 1725 led to the exclusive formation of the deoxy lactone 4a from 1a in high GC-yields (Table 3, entry 2) at all obtained time points. We attempted to achieve comparable shifts in the selectivity towards the dehomologated product 3 within this catalyst family. Nevertheless, increasing the steric bulk near the carbene moiety via introducing again mesityl or diisopropylphenyl on one adjacent nitrogen atom (precatalysts 18 and 19, respectively) did not lead to a change in chemoselectivity, as seen in the thiazolium salt series. Instead we observed a decrease in the conversion to product 4a, indicating that the electronic properties of the triazolium core dominate the steric effects (Table 3, entries 3 and 4). Again, the reaction with the most promising GC-yield was repeated with MeCN as solvent under microwave irradiation, giving lactone in a good isolated yield (5 mmol, upon acetylation, see Scheme 4). With both the ideal substrate/catalyst combinations it was attempted to decrease catalyst loading which leads to a significant decrease in the conversion (see the ESI†).
In summary, we have delivered a clear proof of concept for the principle feasibility of an NHC-controlled intercepted dehomologation of semi-protected carbohydrate derivatives. Herein, we present the first examples of substrate-dependent and – more importantly – catalyst-controlled divergence between selective intercepted dehomologation based on the retro-benzoin reaction on the one hand and the subsequent β-elimination on the other hand. Screening of our catalysts revealed the influence of both steric and electronic properties of carbenes identifying the first ideal substrate/catalyst combinations. Further studies on the scope and limitations of the current methodology are in progress and will deliver an increased understanding of and give rise to more means of exploitation of the fascinating interaction between NHCs and the aldoses’ aldehyde moieties.
We thank T. Blaukovitsch, N. Houszka, Ch. Lim, K. Obleser, M. Schiffrer, K. Schlögl and A. Trpisovsky for technical support. The Austrian Science Fund FWF (Grant P 29138-N34) is gratefully acknowledged for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) T. J. Boltje, T. Buskas and G.-J. Boons, Nat. Chem., 2009, 1, 611 CrossRef CAS PubMed; (b) P. Collins and R. Ferrier, Monosaccharides: Their Chemistry and Their Roles in Natural Products, Wiley, 1995 Search PubMed; (c) E. Jéquier, Am. J. Clin. Nutr., 1994, 59, 682S–685S CrossRef PubMed; (d) A. Varki, Glycobiology, 2017, 27, 3–49 CrossRef CAS PubMed.
- G. J. Boons and K. J. Hale, Organic Synthesis with Carbohydrates, 2008 Search PubMed.
- S. S. Kulkarni, C.-C. Wang, N. M. Sabbavarapu, A. R. Podilapu, P.-H. Liao and S.-C. Hung, Chem. Rev., 2018, 118, 8025–8104 CrossRef CAS PubMed.
- (a) B. O. Fraser-Reid, K. Tatsuta and J. Thiem, Glycoscience: Chemistry and Chemical Biology, Springer Berlin Heidelberg, Berlin, Heidelberg, Berlin, Heidelberg, 2008 CrossRef; (b) R. Mahrwald, Chem. Commun., 2015, 51, 13868–13877 RSC.
- (a) W. H. Binder, R. H. Prenner and W. Schmid, Tetrahedron, 1994, 50, 749–758 CrossRef CAS; (b) J. Gao, R. Haerter, D. M. Gordon and G. M. Whitesides, J. Org. Chem., 1994, 59, 3714–3715 CrossRef CAS; (c) A. Palmelund and R. Madsen, J. Org. Chem., 2005, 70, 8248–8251 CrossRef CAS PubMed; (d) M. Draskovits, C. Stanetty, I. R. Baxendale and M. D. Mihovilovic, J. Org. Chem., 2018, 83, 2647–2659 CrossRef CAS PubMed.
- (a) E. Fischer, Ber. Dtsch. Chem. Ges., 1889, 22, 2204–2205 CrossRef CAS; (b) H. Kiliani, Ber. Dtsch. Chem. Ges., 1885, 18, 3066–3072 CrossRef.
- A. Wohl, Ber. Dtsch. Chem. Ges., 1893, 26, 730–744 CrossRef.
- R. N. Monrad and R. Madsen, Tetrahedron, 2011, 67, 8825–8850 CrossRef CAS.
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef CAS.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed.
- (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed; (b) P.-C. Chiang and J. W. Bode, N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, The Royal Society of Chemistry, 2011, pp. 399–435, 10.1039/9781849732161-00399.
- (a) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC; (b) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719–3726 CrossRef CAS.
- (a) J. Read de Alaniz and T. Rovis, J. Am. Chem. Soc., 2005, 127, 6284–6289 CrossRef CAS PubMed; (b) L. Baragwanath, C. A. Rose, K. Zeitler and S. J. Connon, J. Org. Chem., 2009, 74, 9214–9217 CrossRef CAS PubMed.
- (a) H. Stetter, Angew. Chem., Int. Ed. Engl., 1976, 15, 639–647 CrossRef; (b) A. T. Biju, N. Kuhl and F. Glorius, Acc. Chem. Res., 2011, 44, 1182–1195 CrossRef CAS PubMed; (c) M. Padmanaban, A. T. Biju and F. Glorius, Org. Lett., 2011, 13, 98–101 CrossRef CAS PubMed; (d) C. J. Collett, R. S. Massey, O. R. Maguire, A. S. Batsanov, A. C. O'Donoghue and A. D. Smith, Chem. Sci., 2013, 4, 1514–1522 RSC.
- (a) K. P. Stockton, B. W. Greatrex and D. K. Taylor, J. Org. Chem., 2014, 79, 5088–5096 CrossRef CAS PubMed; (b) B. Kang, T. Sutou, Y. Wang, S. Kuwano, Y. Yamaoka, K. Takasu and K.-i. Yamada, Adv. Synth. Catal., 2015, 357, 131–147 CrossRef CAS.
- S. Wendeborn, R. Mondiere, I. Keller and H. Nussbaumer, Synlett, 2012, 541–544 CrossRef CAS.
- (a) N. T. Reynolds, J. Read de Alaniz and T. Rovis, J. Am. Chem. Soc., 2004, 126, 9518–9519 CrossRef CAS PubMed; (b) K. Y.-K. Chow and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 8126–8127 CrossRef CAS PubMed.
- J. Zhang, C. Xing, B. Tiwari and Y. R. Chi, J. Am. Chem. Soc., 2013, 135, 8113–8116 CrossRef CAS PubMed.
- Y. Zhu, J. Zajicek and A. S. Serianni, J. Org. Chem., 2001, 66, 6244–6251 CrossRef CAS PubMed.
- A. Banchet-Cadeddu, A. Martinez, S. Guillarme, V. Parietti, F. Monneaux, E. Hénon, J.-H. Renault, J.-M. Nuzillard and A. Haudrechy, Bioorg. Med. Chem. Lett., 2011, 21, 2510–2514 CrossRef CAS PubMed.
- M. Becker, F. Liebner, T. Rosenau and A. Potthast, Talanta, 2013, 115, 642–651 CrossRef CAS PubMed.
- (a) X. Bugaut, F. Liu and F. Glorius, J. Am. Chem. Soc., 2011, 133, 8130–8133 CrossRef CAS PubMed; (b) C. G. Goodman and J. S. Johnson, J. Am. Chem. Soc., 2014, 136, 14698–14701 CrossRef CAS PubMed; (c) J. Mahatthananchai and J. W. Bode, Chem. Sci., 2012, 3, 192–197 RSC.
- I. Piel, M. D. Pawelczyk, K. Hirano, R. Fröhlich and F. Glorius, Eur. J. Org. Chem., 2011, 5475–5484 CrossRef CAS.
- Noteworthily, analogous experiments with 3-O-Bn-galactose (probing for the influence of the relative stereochemistry on the rate of the elimination in the redox-lactonisation) gave results closely resembling ones from the gluco-derivative 1a [data not shown].
- D. Enders, K. Breuer, U. Kallfass and T. Balensiefer, Synthesis, 2003, 1292–1295 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc05906g |
| This journal is © The Royal Society of Chemistry 2019 |