A novel crystalline azine-linked three-dimensional covalent organic framework for CO2 capture and conversion†
Abstract
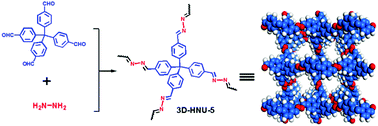
The targeted synthesis of three-dimensional covalent organic frameworks (3D COFs) is a great challenge, especially those synthesized by using a new kind of organic linkage. Herein, for the first time, a novel 3D azine-linked COF (3D-HNU5) has been synthesized and characterized. It is shown that the obtained 3D COF has a 2-fold interpenetrated diamond topology, and shows good chemical/thermal stability and a narrow pore size distribution, which exhibits excellent performance in the selective uptake of CO2 over N2. Moreover, the 3D-HNU5 is found to be an efficient catalyst for the cycloaddition of propargylic alcohols with CO2 into carbonates with excellent catalytic activity under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...