 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A hydrothermally stable ytterbium metal–organic framework as a bifunctional solid-acid catalyst for glucose conversion†
David L.
Burnett
 a,
Ryan
Oozeerally
b,
Ralentri
Pertiwi
c,
Thomas W.
Chamberlain
ab,
Nikolay
Cherkasov
a,
Ryan
Oozeerally
b,
Ralentri
Pertiwi
c,
Thomas W.
Chamberlain
ab,
Nikolay
Cherkasov
 b,
Guy J.
Clarkson
a,
Yuni K.
Krisnandi
b,
Guy J.
Clarkson
a,
Yuni K.
Krisnandi
 c,
Volkan
Degirmenci
c,
Volkan
Degirmenci
 *b and
Richard I.
Walton
*b and
Richard I.
Walton
 *a
*a
aDepartment of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: r.i.walton@warwick.ac.uk
bSchool of Engineering, University of Warwick, Coventry, CV4 7AL, UK. E-mail: v.degirmenci@warwick.ac.uk
cDepartment of Chemistry, Universitas Indonesia, Depok, Indonesia
First published on 5th September 2019
Abstract
Yb6(BDC)7(OH)4(H2O)4 contains both bridging hydroxyls and metal-coordinated waters, possessing Brønsted and Lewis acid sites. The material crystallises from water at 200 °C. Using the solid as a heterogenous catalyst, glucose is converted into 5-hydroxymethylfurfural, via fructose, with a total selectivity of ∼70% after 24 hours at 140 °C in water alone: the material is recyclable with no loss of crystallinity.
The conversion of biomass-derived glucose to organic molecules that may be used as precursors for the production of a variety of useful products for everyday life is important to provide sustainable production of chemicals. 5-Hydroxymethylfurfural (5-HMF) is one such organic molecule formed from glucose, via isomerisation to fructose as a common pathway, and is considered a platform molecule for the formation of a variety of useful chemicals ranging from plastics to drugs to fragrances and agrochemicals.1 The two-step conversion of glucose requires both Lewis and Brønsted acid catalysis. Various studies have probed the possible pathways in solution when homogeneous, solution acids are used as catalysts in aqueous conditions,2–6 and in ionic liquids.7–13 Heterogeneous, solid-acid catalysts would, however, be desirable for this reaction if large-scale processes are to be developed,14,15 avoiding significant amounts of mineral acids and metal salts, with easy of separation of the catalyst from the solution reagents. The use of zeolite-based catalysts that contain both Lewis and Brønsted acid sites has been studied and in particular tin-substituted zeolite beta (Sn-beta) has been shown as a particularly effective catalyst for the isomerisation of glucose into fructose in water,16 or the direct formation of 5-HMF from glucose when a biphasic water/tetrahydrofuran reactor system is used.17 Although Sn-beta is hydrothermally stable at low pH and the solid can be recycled, a significant disadvantage in its use, in particular on an industrial scale, is that the synthesis of the catalyst is a lengthy process requiring the use of seed crystals or a 40 day hydrothermal synthesis reaction using hydrofluoric acid, an extremely toxic and corrosive substance.16 Furthermore, in continuous flow reactors Sn-beta can be unstable and is rapidly deactivated.18
Metal–organic frameworks have been considered as solid acid catalysts.19 The dehydration of glucose to 5-HMF using MIL-101(Cr) functionalised with sulfonic acid groups (–SO3H) was reported by Herbst and Janiak in 2016.20 The highest 5-HMF yield of 29% was obtained at 130 °C with a 5 hour reaction time in tetrahydrofuran![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (39
water (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as solvent. Su et al. studied the same catalyst and found the highest conversions in mixed organic/aqueous solvent systems and demonstrated the effectiveness of the solid in a fixed-bed, continuous reactor.21 MIL-101(Cr)–SO3H showed high efficiency with more than 90% yield of 5-HMF when DMSO was used as solvent.22 A composite of MIL-101(Cr) and chromium hydroxide showed optimum isomerisation of glucose into fructose in ethanol as solvent.23 Other MOFs that have been used for glucose conversion include the zirconium-based NU-1000, with phosphate modification to induce Lewis acidity,24 and the zirconium material UiO-66 with sulfonyl-modified ligands and inherent Lewis acidity from defects notably allowing conversion of glucose to fructose, along with a significant amount of 5-HMF in water alone,25 or in alcohols as solvents.26 While MIL-101 is constructed from the cheap and benign ligand benzene-1,4-dicarboxylate, the metal Cr is known to be toxic to humans and harmful to the environment; although these detrimental properties are largely associated with the +6 oxidation state, Cr3+ is considered an irritant, and its release into the environment is clearly undesirable if it were to encounter oxidising conditions.27 Replacement of the chromium in MIL-101 by other trivalent cations leads to an instability of the material,28 and we have therefore explored the chemistry of water-stable metal–organic frameworks containing more benign metals. Herein, we focus on materials that contain Yb3+ as the metal centre, with several reasons for the choice of this metal: (i) ytterbium triflate is well-known Lewis acid used for various organic transformations,29 (ii) YbCl3 in solution has already been used as a homogeneous Lewis acid catalysts for glucose conversion,30,31 and (iii) metal–organic frameworks based on the rare-earth cations are known to be relatively chemically and thermally robust, as well as showing a wide structural variety.32
1) mixture as solvent. Su et al. studied the same catalyst and found the highest conversions in mixed organic/aqueous solvent systems and demonstrated the effectiveness of the solid in a fixed-bed, continuous reactor.21 MIL-101(Cr)–SO3H showed high efficiency with more than 90% yield of 5-HMF when DMSO was used as solvent.22 A composite of MIL-101(Cr) and chromium hydroxide showed optimum isomerisation of glucose into fructose in ethanol as solvent.23 Other MOFs that have been used for glucose conversion include the zirconium-based NU-1000, with phosphate modification to induce Lewis acidity,24 and the zirconium material UiO-66 with sulfonyl-modified ligands and inherent Lewis acidity from defects notably allowing conversion of glucose to fructose, along with a significant amount of 5-HMF in water alone,25 or in alcohols as solvents.26 While MIL-101 is constructed from the cheap and benign ligand benzene-1,4-dicarboxylate, the metal Cr is known to be toxic to humans and harmful to the environment; although these detrimental properties are largely associated with the +6 oxidation state, Cr3+ is considered an irritant, and its release into the environment is clearly undesirable if it were to encounter oxidising conditions.27 Replacement of the chromium in MIL-101 by other trivalent cations leads to an instability of the material,28 and we have therefore explored the chemistry of water-stable metal–organic frameworks containing more benign metals. Herein, we focus on materials that contain Yb3+ as the metal centre, with several reasons for the choice of this metal: (i) ytterbium triflate is well-known Lewis acid used for various organic transformations,29 (ii) YbCl3 in solution has already been used as a homogeneous Lewis acid catalysts for glucose conversion,30,31 and (iii) metal–organic frameworks based on the rare-earth cations are known to be relatively chemically and thermally robust, as well as showing a wide structural variety.32
At least three different ytterbium MOFs crystallise from N,N-dimethylformamide (DMF)–water solutions of Yb3+ and benzene-1,4-dicarboxylic acid (H2BDC), depending on the solvent composition and temperature.33,34 This was the starting point for considering materials that might be water stable and thus the hydrated material Yb2(BDC)3(DMF)2(H2O)2 (1) was selected, Fig. 1a, that crystallises from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 DMF
50 DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions at 100 °C34 to investigate its behaviour in pure water. Hydrothermal treatment of (1) at 200 °C (ESI†) produced single crystals of Yb6(BDC)7(OH)4(H2O)4 (2), whose structure, determined by single crystal X-ray diffraction was found to be analogous to yttrium and ytterbium materials reported by Weng et al.35 The structure of (2) is completely different to (1). The latter, Fig. 1a contains dimers of 7-coordinate Yb3+ centres bridged the dicarboxylates, crosslinked by further BDC linkers to give α-Po type net that is interpenetrated with another such net, while (2) contains three distinct 8-coordinate Yb3+ centres bridged by hydroxides and BDC, two of which have terminal waters, with BDC ligands crosslinking denser regions of the structure, Fig. 1b. The building unit of the structure is a hexameric cluster, shown on Fig. 2, where six Yb centres are linked therein directly by shared oxygens. We also discovered that by moderating the synthesis conditions (see ESI†) the novel salt Yb2(BDC)3 (3) crystallised directly from water at 200 °C. The structure of (3) contains isolated Yb centres, bridged by carboxylate functions of the BDC in a Z,Z-μ2-η1:η1 mode, to give extended chains, BDC, Fig. 1c. The chains run in two perpendicular directions, to give a pseudo-layered structure, that are crosslinked by the bridging BDC ligands.
water solutions at 100 °C34 to investigate its behaviour in pure water. Hydrothermal treatment of (1) at 200 °C (ESI†) produced single crystals of Yb6(BDC)7(OH)4(H2O)4 (2), whose structure, determined by single crystal X-ray diffraction was found to be analogous to yttrium and ytterbium materials reported by Weng et al.35 The structure of (2) is completely different to (1). The latter, Fig. 1a contains dimers of 7-coordinate Yb3+ centres bridged the dicarboxylates, crosslinked by further BDC linkers to give α-Po type net that is interpenetrated with another such net, while (2) contains three distinct 8-coordinate Yb3+ centres bridged by hydroxides and BDC, two of which have terminal waters, with BDC ligands crosslinking denser regions of the structure, Fig. 1b. The building unit of the structure is a hexameric cluster, shown on Fig. 2, where six Yb centres are linked therein directly by shared oxygens. We also discovered that by moderating the synthesis conditions (see ESI†) the novel salt Yb2(BDC)3 (3) crystallised directly from water at 200 °C. The structure of (3) contains isolated Yb centres, bridged by carboxylate functions of the BDC in a Z,Z-μ2-η1:η1 mode, to give extended chains, BDC, Fig. 1c. The chains run in two perpendicular directions, to give a pseudo-layered structure, that are crosslinked by the bridging BDC ligands.
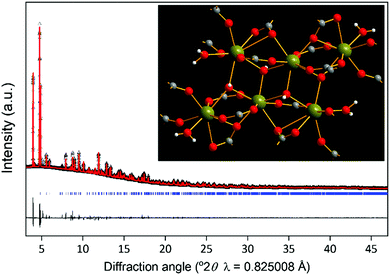 | ||
| Fig. 2 Powder XRD Le Bail fit of a bulk sample of Yb6(BDC)7(OH)4(H2O)4 (2) (see ESI† for crystal structure parameters). The data points are black triangles, the fitted pattern the red line, the difference curve the grey line and the blue ticks the positions of allowed Bragg peaks. The inset shows a view of the hexameric cluster unit in the material, with the same atom key as for Fig. 1, except for inclusion of hydrogen atoms as white spheres. | ||
The synthesis of phase-pure (2), was subsequently achieved by addition of extra YbCl3·6H2O to provide the correct ratio of Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDC as required by the chemical composition (see ESI†), Fig. 2. We also considered the possibility of inclusion of a Brønsted acid functionality by use of the sulfonic-acid modified 2-monosulfo-benzene-1,4-dicarboxylate (MSBDC) linker, using the sodium salt of the linker as precursor, as has found to be previously successful for modifying MOFs such as UiO-66 and MIL-101. This, however, yielded a novel crystalline structure only when DMF was used as solvent, [Yb(MSBDC)(DMF)2] (4), and in this material the sulfonic acid functionality is deprotonated and coordinated to the Yb3+ centres, along with the carboxylate functionalities to yield a rather dense coordination polymer (ESI†). The material (4) was also found to be unstable under hydrothermal conditions, dissolving completely upon being heated in water above 100 °C.
BDC as required by the chemical composition (see ESI†), Fig. 2. We also considered the possibility of inclusion of a Brønsted acid functionality by use of the sulfonic-acid modified 2-monosulfo-benzene-1,4-dicarboxylate (MSBDC) linker, using the sodium salt of the linker as precursor, as has found to be previously successful for modifying MOFs such as UiO-66 and MIL-101. This, however, yielded a novel crystalline structure only when DMF was used as solvent, [Yb(MSBDC)(DMF)2] (4), and in this material the sulfonic acid functionality is deprotonated and coordinated to the Yb3+ centres, along with the carboxylate functionalities to yield a rather dense coordination polymer (ESI†). The material (4) was also found to be unstable under hydrothermal conditions, dissolving completely upon being heated in water above 100 °C.
The coordination polymers (2) and (3) both crystallise in water above 200 °C in 3 day synthesis reactions, so are extremely hydrothermally stable materials. For (2) the structure has channels containing occluded water, and thermogravimetric and thermodiffraction analysis reveals zeolitic behaviour with removal of water on heating to 200 °C (ESI†). Importantly, (2) has the potential for solid-acid properties with both bridging hydroxyls (Brønsted acidity) and coordinatively unsaturated Yb3+ once bound water is removed to lower the coordination number to seven (Lewis acidity), Fig. 2. In contrast (3) is a dense structure with fully saturated Yb centres, containing only the linker and the metal cation with no occluded species or additional ligands. We therefore chose (2) as a potential water-stable solid-acid catalyst, and examined the conversion of glucose in deionised water (pH = 7) at 140 °C, with the results summarised in Fig. 3 (see ESI† for full data). For comparison, we studied an aqueous solution of YbCl3 as a homogeneous catalyst and Yb2O3 as a heterogeneous comparison (with the same total Yb content used for each). While the homogeneous solution of YbCl3 gives the highest conversion of glucose, the selectivity towards fructose and 5-HMF is not high (48.3%); in fact previous work has shown that the pH needs to be lowered to obtain more significant conversion to the desired products from metal chloride salts, and then only at 170 °C using a biphasic solvent mixture.30,31 Solid Yb2O3 shows a low conversion and only a small amount of fructose with no detectable 5-HMF in the product mixture after 3 hours, as expected in the absence of any Brønsted acid sites. In contrast (2) shows a reasonable conversion of glucose (∼16%) after 3 hours and a selectivity towards fructose and 5-HMF of 50%. Since it may be expected that the kinetics of the heterogeneous reaction may be retarded compared to the homogeneous case, we examined long reactions. As can be seen on Fig. 3, extending the reaction time to 24 hours with (2), increases the conversion to a useful 28%, with a notable 65% selectivity towards 5-HMF, and 70% total selectivity towards fructose and 5-HMF. In all cases only negligible amounts of mannose are produced, a possible byproduct from the epimerisation of glucose.
 | ||
| Fig. 3 Results of catalytic conversion of glucose using [Yb6(BDC)7(OH)4(H2O)4] (2) and compared with a homogeneous solution of YbCl3 and with solid Yb2O3. | ||
The presence of acid sites in (2) was proven using ammonia temperature programmed desorption, which is consistent with the presence of two types of acid sites associated with the material, and pyridine adsorption that indicates both Brønsted and Lewis acid sites (ESI†). The fact that a significant amount of 5-HMF is produced, and that the proportion of this vs. fructose increases with extended reaction time, implies the presence of Brønsted acidity inherent to the material. Interestingly, lowering the pH to 2.5 with HCl offers no advantage: as shown on Fig. 3, the total conversion and distribution of products is similar to at pH = 7 at short reaction times, while after 24 hours the higher conversion of glucose is associated with a much lower selectivity towards the desired products, suggesting decomposition of glucose by other pathways.
We examined the possibility of recycling the catalyst, and after recovering the solid, drying and adding to a fresh reaction, virtually the same conversions and selectivities were obtained over four runs, Fig. 4a. SEM shows little change in the particle morphology before and after use, with a distribution of crystallites micron-sized and less (Fig. 4b and c), while powder XRD, Fig. 4d, shows that the material remains intact after use as a catalyst, albeit contaminated with some amorphous humins as byproducts, consistent with the brown colour of the material after catalysis.
 | ||
| Fig. 4 (a) Recyclability of the catalyst (2) with SEM (b) before and (c) after heating in glucose at 140 °C for 24 hours (the scale bar is 1 μm) and (d) powder XRD (λ = 1.5406 Å) after reuse. | ||
The ytterbium MOF (2) acts as a catalyst for glucose conversion in water alone, with no additional mineral acid necessary. The fact that significant amounts of 5-HMF are produced under these conditions, and is indeed the majority product after 24 hours, clearly indicates that the solid provides the necessary Brønsted acidity. Most other MOFs that have been reported so far have been studied in mixed water/organic solvents or with the addition of HCl (see Table S4, ESI†), but for the cases where just water has been used the ytterbium MOF outperforms the zirconium MOF NU-1000, which gave 60% conversion of glucose but yields of only 19% fructose and 2.3% 5-HMF (at 140 °C for 5 hours).24 Sulfonyl-modified UiO-66 gave a similar 35.9% conversion to the Yb MOF in water alone and an initially high selectivity, but the efficiency dropped on recycling the solid.25 Comparisons with other solid acids used for glucose shows that our materials offer significant advantages. For example, conventional solid-acid catalysts, such as sulfated zirconium oxide,36 or sulfated niobium oxide,37 require concentrated mineral acids, or other noxious reagents, for their synthesis. Tsapatsis and co-workers have recently shown that a composite of MIL-101(Cr) and Cr(OH)3 gave high conversion of glucose but this was in a two-step process with ethanol as the solvent for isomerisation to ethyl fructoside, followed by addition of water to allow hydrolysis to fructose, each performed at 100 °C for 24 hours with almost 60% of the product fructose.23 The use of multiple batch reaction steps requiring long reaction times is likely to be less attractive than continuous flow systems on scale-up. In fact, a lower conversion with higher selectivity may be useful if applied within continuous flow recycle reactors. Further work is needed to understand the molecular-scale mechanisms of the conversions of sugars over MOF catalysts: this includes the identity of intermediates and byproducts, and the possibility of disassembly of the surface of the MOF. This would then allow optimisation of activity and implementation of the materials in real processes. The use of hydrothermally stable materials containing the smaller lanthanide cations in heterogeneous solid-acid catalysis will also be of wider relevance to other industrial conversions, such as condensations and dehydrations, and applications where stability in water is needed,38 for which metal–organic frameworks are only beginning to be being explored.39,40
We thank the Royal Society (Challenge Grant CH160099) and the EPSRC grant EP/P511432/1, Global Challenge Research Fund (GCRF) Institutional Award for the University of Warwick, for funding. Support from a Research Grants for Higher Education 2016 from DGHE, Indonesia is also gratefully acknowledged (No. 1136/UN2.R12/HKP/05.00/2016). The I11 beamtime was obtained through the Diamond Light Source Block Allocation Group award “Oxford Solid State Chemistry BAG to probe composition–structure–property relationships in solids” (EE13284) and we thank Dr Mark Senn and Mr Gabriel Clarke for their assistance. The research data supporting this publication can be accessed at: https://wrap.warwick.ac.uk/124044.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. B. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- D. W. Harris and M. S. Feather, J. Am. Chem. Soc., 1975, 97, 178–182 CrossRef CAS.
- F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2007, 46, 7703–7710 CrossRef CAS.
- V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997–4006 CrossRef CAS.
- P. Korner, D. Jung and A. Kruse, Green Chem., 2018, 20, 2231–2241 RSC.
- A. A. Marianou, C. M. Michailof, A. Pineda, E. F. Iliopoulou, K. S. Triantafyllidis and A. A. Lappas, Appl. Catal., A, 2018, 555, 75–87 CrossRef CAS.
- S. Q. Hu, Z. F. Zhang, J. L. Song, Y. X. Zhou and B. X. Han, Green Chem., 2009, 11, 1746–1749 RSC.
- X. L. Tong and Y. D. Li, ChemSusChem, 2010, 3, 350–355 CrossRef CAS.
- T. Stahlberg, S. Rodriguez-Rodriguez, P. Fristrup and A. Riisager, Chem. – Eur. J., 2011, 17, 1456–1464 CrossRef CAS.
- H. Lei, S. Yong and L. Lu, Prog. Chem., 2012, 24, 483–491 Search PubMed.
- E. A. Pidko, V. Degirmenci and E. J. M. Hensen, ChemCatChem, 2012, 4, 1263–1271 CrossRef CAS.
- E. A. Pidko, V. Degirmenci, R. A. van Santen and E. J. M. Hensen, Angew. Chem., Int. Ed., 2010, 49, 2530–2534 CrossRef CAS.
- E. A. Pidko, V. Degirmenci, R. A. van Santen and E. J. M. Hensen, Inorg. Chem., 2010, 49, 10081–10091 CrossRef CAS.
- V. Degirmenci and E. J. M. Hensen, Environ. Prog. Sustainable Energy, 2014, 33, 657–662 CrossRef CAS.
- V. Degirmenci, E. A. Pidko, P. C. M. M. Magusin and E. J. M. Hensen, ChemCatChem, 2011, 3, 969–972 CrossRef CAS.
- M. Moliner, Y. Roman-Leshkov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6164–6168 CrossRef CAS.
- E. Nikolla, Y. Roman-Leshkov, M. Moliner and M. E. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS.
- D. Padovan, C. Parsons, M. S. Grasina and C. Hammond, Green Chem., 2016, 18, 5041–5049 RSC.
- Y. Zhang, V. Degirmenci, C. Li and E. J. M. Hensen, ChemSusChem, 2011, 4, 59–64 CrossRef CAS.
- A. Herbst and C. Janiak, New J. Chem., 2016, 40, 7958–7967 RSC.
- Y. Su, G. G. Chang, Z. G. Zhang, H. B. Xing, B. G. Su, Q. W. Yang, Q. L. Ren, Y. W. Yang and Z. B. Bao, AIChE J., 2016, 62, 4403–4417 CrossRef CAS.
- J. Z. Chen, K. G. Li, L. M. Chen, R. L. Liu, X. Huang and D. Q. Ye, Green Chem., 2014, 16, 2490–2499 RSC.
- Q. Guo, L. M. Ren, P. Kumar, V. J. Cybulskis, K. A. Mkhoyan, M. E. Davis and M. Tsapatsis, Angew. Chem., Int. Ed., 2018, 57, 4926–4930 CrossRef CAS.
- M. Yabushita, P. Li, T. Islamoglu, H. Kobayashi, A. Fukuoka, O. K. Farha and A. Katz, Ind. Eng. Chem. Res., 2017, 56, 7141–7148 CrossRef CAS.
- R. Oozeerally, D. L. Burnett, T. W. Chamberlain, R. I. Walton and V. Degirmenci, ChemCatChem, 2018, 10, 706–709 CrossRef CAS.
- M. D. de Mello and M. Tsapatsis, ChemCatChem, 2018, 10, 2417–2423 CrossRef.
- A. D. Dayan and A. J. Paine, Hum. Exp. Toxicol., 2001, 20, 439–451 CrossRef CAS.
- R. Pertiwi, R. Oozeerally, D. L. Burnett, T. W. Chamberlain, N. Cherkasov, M. Walker, R. J. Kashtiban, Y. K. Krisnandi, V. Degirmenci and R. I. Walton, Catalysts, 2019, 9, 437 CrossRef CAS.
- L. J. Zhang, H. L. Lu, Z. W. Wu and Y. S. Huang, Curr. Org. Chem., 2013, 17, 2906–2920 CrossRef CAS.
- Y. J. Pagan-Torres, T. F. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef CAS.
- T. F. Wang, J. A. Glasper and B. H. Shanks, Appl. Catal., A, 2015, 498, 214–221 CrossRef CAS.
- C. Pagis, M. Ferbinteanu, G. Rothenberg and S. Tanase, ACS Catal., 2016, 6, 6063–6072 CrossRef CAS.
- Y. Wu, M. I. Breeze, G. J. Clarkson, F. Millange, D. O'Hare and R. I. Walton, Angew. Chem., Int. Ed., 2016, 55, 4992–4996 CrossRef CAS.
- M. I. Breeze, T. W. Chamberlain, G. J. Clarkson, R. P. de Camargo, Y. Wu, J. F. de Lima, F. Millange, O. A. Serra, D. O'Hare and R. I. Walton, CrystEngComm, 2017, 19, 2424–2433 RSC.
- D. F. Weng, X. J. Zheng and L. P. Jin, Eur. J. Inorg. Chem., 2006, 4184–4190 CrossRef CAS.
- H. P. Yan, Y. Yang, D. M. Tong, X. Xiang and C. W. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS.
- E. L. S. Ngee, Y. J. Gao, X. Chen, T. M. Lee, Z. G. Hu, D. Zhao and N. Yan, Ind. Eng. Chem. Res., 2014, 53, 14225–14233 CrossRef CAS.
- S. Yuan, L. Feng, K. C. Wang, J. D. Pang, M. Bosch, C. Lollar, Y. J. Sun, J. S. Qin, X. Y. Yang, P. Zhang, Q. Wang, L. F. Zou, Y. M. Zhang, L. L. Zhang, Y. Fang, J. L. Li and H. C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef.
- Z. G. Hu and D. Zhao, CrystEngComm, 2017, 19, 4066–4081 RSC.
- J. C. Jiang and O. M. Yaghi, Chem. Rev., 2015, 115, 6966–6997 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis details, crystallographic information and further experimental characterisation. CCDC 1892235 (3), 1892236 (4), 1892237 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc05364f |
| This journal is © The Royal Society of Chemistry 2019 |

