Vapour-phase deposition of oriented copper dicarboxylate metal–organic framework thin films†
Abstract
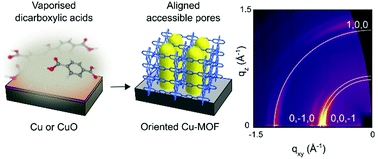
Copper dicarboxylate metal–organic framework films are deposited via chemical vapour deposition. Uniform films of CuBDC and CuCDC with an out-of-plane orientation and accessible porosity are obtained from the reaction of Cu and CuO with vaporised dicarboxylic acid linkers.


 Please wait while we load your content...
Please wait while we load your content...