Open Access Article
Open Access ArticleNanobiohybrids: a new concept for metal nanoparticles synthesis
Jose M.
Palomo

Department of Biocatalysis, Institute of Catalysis (CSIC), Marie Curie 2, Cantoblanco, UAM Campus, 28049, Madrid, Spain. E-mail: josempalomo@icp.csic.es
First published on 19th July 2019
Abstract
In recent years, nanoscience and nanotechnology have brought a great revolution in different areas. In particular, the synthesis of transition metal nanoparticles has been of great relevance for their use in areas such as biomedicine, antimicrobial properties or catalytic applications for chemical synthesis. Recently, an innovative straightforward and very efficient synthesis of these nanoparticles by simply using enzymes as inductors in aqueous media has been described. This represents a very green alternative to the different methodologies described in the literature for metal nanoparticles preparation where harsh conditions are necessary. In this review the most recent advances in the synthesis of metal nanoparticles by this green technology, explaining the synthetic mechanism, the role of the enzyme in the formation of the nanoparticles and the effect on the final properties of these nanoparticles, are summarised. The application of these novel metal nanoparticles–enzyme hybrids in synthetic chemistry as heterogeneous catalysts with metal or dual (enzymatic and metallic) activity and their capacity as environmental and antimicrobial agents have also been discussed.
1. Introduction
Transition metal nanoparticles (Me NPs) have been successfully developed for years with interesting applications as catalysts, imaging reagents and magnetic materials in bionanotechnology and biomedicine.1–3However, the main question in the synthetic strategies is focused on the development of methodologies that allow the creation of these nanomaterials in a simple, efficient and sustainable way.
For this reason, researchers have started to apply biological entities as an elegant approach for the direct synthesis of metal nanoparticles. This biological entity has the ability to induce the formation of these nanoparticles, sometimes even controlling the size and structural shape, and avoiding aggregation problems.4,5
A very important issue is the selection of the biological entity. One of the main strategies described in the literature for this green synthetic approach is based on the use of microorganisms.6–9 Entire biological units such as prokaryotic or eukaryotic microorganisms have been employed for the preparation of nanoparticles of different metals (Au, Ag, Cd, Pt, Zn and Fe3O4) under moderate pressures and temperatures. The advantage is that microorganisms secrete large quantities of enzymes, which participate in the enzymatic reduction of metal ions.10 However, the control, localization and morphology of the nanoparticles depend on the micro-organism species used because of the different mixtures of enzymes and proteins that can be involved in the process, generating nanoparticles of different sizes.11
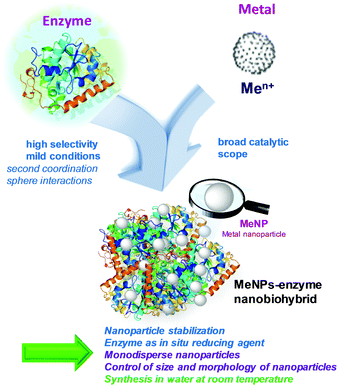
In addition, many examples show that the nanoparticles synthesized in this way are not monodisperse, in many cases have large sizes and are obtained at very low rates.12 Therefore, the most appropriate strategy consists in the use of isolated proteins or enzymes. Protein scaffolds provide unique metal coordination environments that promote biomineralization processes and allow control of size, control particle growth and prevent aggregation among the produced nanoparticles.13,14 At this point, it has been reported in the literature examples where the use of proteins makes the biofabrication of metal nanoparticles13,14 or nanoclusters14f,g possible. However, the application of enzymes (biological catalysts) opens a new concept, where synthetic biohybrids combine a three-dimensional environment with high selectivity (by the enzyme) with a wide range of catalysis (by the synthesized metal nanoparticles) (Fig. 1), which represents an exceptional possibility to create materials with multiple catalytic activities. These materials could also represent a new strategy in the world of artificial metalloenzymes, where the methodologies currently used to create new catalytic activities are based mainly on the substitution of metals in the natural source, and the insertion of the organometallic complex in the active site or binding pocket in a particular protein or enzyme.15
In this way, the use of commercially available enzymes, or even genetically engineered enzymes, which would make the method more reproducible with production in higher amounts, is mandatory for a successful and applicable strategy. The pioneer strategy was first developed using a lipase from Candida antarctica (fraction B) (CAL-B),16 a 33 kDa monomeric enzyme with a size of 3 nm × 4 nm × 5 nm, which is commercially available from Novozymes. This enzyme, probably the most used lipase in biotransformations,17 has a particular catalytic cavity that gives it a very broad catalytic performance in terms of chemical groups and chemical reactions.
Therefore, highly dispersed ultra-small precious metal (Ag, Pd or Au) nanoparticles in situ generated and embedded on the CAL-B protein structure were synthesized in aqueous media and at room temperature.16
The methodology for creating heterogeneous enzyme–metal nanoparticles nanobiohybrids has also recently been extended to other enzymes, native and also genetically or chemically modified.18–32 This straightforward and environmentally benign technology allows the creation of biohybrid materials in a multi-milligram scale. One of the main advantages of these biohybrid materials is their catalytic applications. These represent the perfect example of artificial metaloenzymes33 in heterogeneous form, which, in fact, is poorly developed.34
The heterogeneous nanomaterials, with metal catalytic activity in an enzyme network that conserves its biological activity, present the advantage of acting as metallic or dual (metallic and enzymatic activity) catalysts,16,18,21 which is of enormous relevance for cascade processes.35
This review will discuss the synthetic mechanism and the role of the enzyme structure on the final synthetic nanomaterial, also showing the practical application of these enzyme–metal nanoparticles nanobiohybrids in different research fields.
2. Synthesis of metal nanoparticles induced by enzymes: nanobiohybrids
Enzymes from different sources (bacteria, yeast, and fungi), obtained by recombinant methods and also with different sizes have been successfully applied to create metal nanoparticles (Table 1).16,18–32| Nano hybrid | Enzyme (mg mL−1) | MS/MNPsl (mM/nm) | RCm | T | Time (h) | Ref.t |
|---|---|---|---|---|---|---|
| a β-Galactosidase from A. oryzae. b Keratinase from S. maltophilia r13. c β-Glucosidase from T. petrophila GH1. d β-Glucosidase from T. petrophila GH3. e Lipase from Candida antarctica B. f Lysozyme chicken egg white. g Esterase from M. smegmatis. h Lipase from C. rugosa. i Thermoalkalophilic lipase from Geobacillus thermocatenulatus (GTL) genetically modified in Ala193 to Cys and chemically modified with peptide (p: Ac-Cys-Phe-Gly-Phe-Gly-Phe-CONH2). j Lignin peroxidase SW30. k Glucose oxidase from A. niger. l MS: metal salt concentration (mM), MNPs: metal nanoparticles size (nm). m RC: reaction conditions. n S: co-solvent. o D: dimethylformamide (DMF). p P/T: PEI/Triton X-100. q PB: sodium phosphate buffer. r T: temperature (°C). s O.N: overnight. t Ref.: reference. | ||||||
| Enzyme-AgNPs | β-Gala (102 kDa) (1 mg mL−1) | AgNO3 (100/13) | AcONa pH 4.5 | 37 | 36 | 25 |
| Kerb (35 kDa) | AgNO3 (2/8.4) | H2O pH 9 | 40 | 24 | 22a | |
| GluGH1c (104 kDa) (0.1 mg mL−1) | AgNO3 (1/53) | TrisHCl pH 8 (UV) | r.t. | 1 | 30 | |
| GluGH3d (160 kDa) (0.1 mg mL−1) | AgNO3 (1/48) | TrisHCl pH 8 (UV) | r.t. | 1 | 30 | |
| CALBe (33 kDa) (0.5 mg mL−1) | AgNO3 (120/8) | H2O | r.t. | 24 | 16 | |
| Enzyme-PtNPs | Lysf (14.3 kDa) (5 mg mL−1) | HPtCl6 (10/<1) | NaOH | 37 | O.N.s | 20b |
| Estg (189 kDa) (5 ng mL−1) | K2PtCl4 (0.5–3/2) | HEPES pH 8 NaBH4 | r.t. | 1 h | 20a | |
| Enzyme-PdNPs | CAL-Be (0.5 mg mL−1) | Pd(OAc)2 (22/2) | H2O 20%Sn | r.t. | 24 | 16 |
| CRLh (63 kDa) (1 mg mL−1) | Pd(OAc)2 (7.3/6–8) | H2O 20Do (PEI) | r.t. | 24 | 21 | |
| CRL (63 kDa) (1 mg mL−1) | Pd(OAc)2 (7.3/9–24) | H2O 20Do P/Tp | r.t. | 24 | 21 | |
| GTLi (43 kDa), (0.4 mg mL−1) | Pd(OAc)2 (22/3) | H2O 20Do | r.t. | 24 | 18 | |
| Enzyme-AuNPs | Peroxj (97 kDa) (0.3 mg mL−1) | HAuCl4 (1/10) | PBq pH 7.4 | 37 | 0.2 | 23a |
| GOXk (160 kDa) (0.7 mg mL−1) | HAuCl4 (600/9.9) | PBq pH 7 | 37 | 36 | 23b | |
| CAL-B (0.5 mg mL−1) | HAuCl4 (60/8) | H2O | r.t. | 24 | 16 | |
| Lysf (1–25 mg mL−1) | HAuCl4 (10/13) | NaOH | 37 | O.N. | 20b | |
Transition metal nanoparticles such as Pd(0), Pt(0), Au(0) and Ag(0) have been synthesized by this technology.
The strategy is very simple and straightforward, and consists of the addition of a metal salt in a solution containing an enzyme dissolved directly in distilled water or in buffer at a particular temperature, generally from room temperature to 40 °C (Fig. 1 and Table 1). After around 30 minutes to one hour, depending on each case, the solution goes from a transparent to a cloudy state, which indicates the aggregate formation, the initial step until the final nanobiohybrid generation. This mixture is maintained for the corresponding time (Table 1) and then centrifuged recovering the well-formed aggregate. Table 1 shows the different examples currently described in the literature in the preparation of nanobiohybrids. In all cases, enzymes play a critical role in the final nanoparticles preparation. Enzymes act as the stabilizer, reducing agent and inductor of very well-dispersed small metal nanoparticles formation (Fig. 1). The three-dimensional structure of the enzyme acts as a scaffold of the in situ generated NPs for obtaining the final heterogeneous material. This methodology differs clearly from the many examples of immobilization of enzymes in metal nanoparticles.36
Different parameters have been found to be quite important to obtain a particular size and morphology of the precious metal nanoparticles on the nanobiohybrid.
The protein dimension was one of them. From Table 1 it is possible to observe that, in general, as the molecular weight of the protein increases, the size of the nanoparticles also increases under similar experimental conditions. For example, in the preparation of AgNPs, the diameter of the nanoparticles changed from 8 nm using CAL-B (33 kDa) to 12 nm using beta-galactosidase (102 kDa). Larger Ag nanoparticles of around 50 nm were obtained using homodimeric glucosidases (>100 kDa) in a photocatalytic process (Table 1).
In addition, the density of the anchoring sites present in the enzyme (amino or carboxylic groups) on its surface should be more efficient in immobilizing metal species.
Another parameter was the presence of additives.21 This is of particular importance when the stability of the enzyme used in the preparation of the hybrid is low. For example, using C. rugosa lipase and polyethylenimine (PEI) of Mw 800 Da, biohybrids containing Pd nanoparticles with a diameter of 6–8 nm were obtained, whereas the additional presence of Triton X-100, a detergent, caused the increase in the size of the nanoparticles (up to 24 nm), probably due to the coating of this detergent around the protein structure, which increases the final size of the protein (Table 1). However, in the AuNPs, the pH solution was important; larger nanoparticles were formed using lyzosyme (14 kDa), about 13 nm under basic conditions, whereas smaller nanoparticles (10 nm diameter size) were obtained using glucose oxidase (160 kDa) in phosphate buffer at pH 7 (Table 1). Lysozyme was also used to successfully synthesize Pt nanoclusters (PtNCs) with a diameter size of <1 nm (Table 1).20b These examples also show that the experimental conditions have influenced the final results in addition to the structural characteristics of the enzymes.
In addition, proteins with high thermal and solvent stability appear to be very good candidates for final highly stable bionanohybrids.18
Another advantage of this technique is that the enzymatic activity still continues in the heterogeneous nanohybrids, for example, more than 50% of the enzymatic activity was conserved in the biohybrid CAL-B-PdNPs,16 or more than 72% of the GH1 glucosidase activity was conserved in Glu-AgNPs (Table 1).30
The preparation of metal NPs using enzymes to synthesize bionanohybrids also offers the possibility of achieving a heterogeneous material with double catalytic activity (metallic and enzymatic). This is an important issue in cascade reaction processes in which both catalytic activities can be worked in a synergistic way to finally produce the desired product in a faster and more cost-efficient manner.35
Therefore, this strategy, although still in its development, seems to be proof that we can select the type and size of metal nanoparticles obtained.
2.1 Discussion on metal nanoparticles formation mechanism
One of the main key processes in the generation of the metal bionanohybrids is how metal nanoparticles are formed. In relation to this, as mentioned above, the structure of the protein plays an extremely important role by finally allowing the in situ generation of crystalline metal nanoparticles well dispersed in the protein network, avoiding the common problem of aggregation. Moreover, the proposed mechanism for the formation of this enzyme–metal nanoparticles biohybrid consists of three different steps, (i) the ionic binding of metal (Me) ions with different amino acid residues of the protein, (ii) nucleation, reduction of Men+ to Me(0) and (iii) coalescence, the growth of Me nanoparticles (Fig. 2).16,27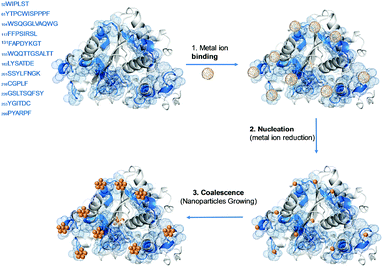 | ||
| Fig. 2 Proposed mechanism of metal nanoparticles formation. As an example, the sequence and structure of CAL-B. | ||
In order to understand the mechanism, it is mandatory to consider the role of aminoacids and small peptide sequences of the protein, which are involved in the entire nanobiohybrid formation.
The first step consists in a rapid adsorption of soluble Men+ ions on the peptide sequences of the enzyme (intermediate binding affinity), reducing its solubility and acting as a cross-linker between the enzyme's molecules (initial fast precipitation).
For a successful step two, these peptides would possess adequate reducing power and the ability to bring the metal ions closer to the reduction sites without excessive chelation, which inactivates the metal ions.
These phenomena (metal binding and reducing ability) of free amino acids and tailor-made synthetic small peptides has been deeply investigated by Wang and coworkers.37 Many parameters have been analyzed finally getting to some general rules that mainly indicate the precise amino acid sequence composition as a key-element to obtain the formation of nanomaterials.37
Contrary to the common practice of relying on reaction macroenvironment adjustments (e.g., pH, temperature and reactant concentration) for kinetic control, the peptide synthesis of metal nanoparticles delivers more precise kinetic control at the reaction microenvironment level by dialing in different amino acid sequences for the right combination of reducing, capping, and morphogenic functions. In proteins (polypeptide chains) the increasing complexity of the peptide sequence and the increasing possibility for structure and conformation variations may also contribute to the overall functions of the peptides.
In general for a successful formation of nanoparticles, the protein should contain particular peptide sequences, the so called ideal peptides, which must be formed by aminoacids with moderate binding affinity for both metal ions and prepared metal particles (i.e. amino acids presenting hydrophobic or charged side chains, with opposite charges to that shown by the metal ions) together with neighboring amino acids showing a strong reducing ability (tryptophans, serines, threonines, tyrosines or cysteines).
In particular, the CAL-B lipase sequence and tridimensional structure were analyzed searching for some naturally present binding/reducing peptide sequences. Fig. 2 shows several possible peptides (in blue) containing adequate aminoacids for the first binding metal ions and the subsequent in situ reduction. Furthermore, after the analysis of the whole sequence it was possible to find Trp (5), Tyr (9), Ser (31) and Thr (37) involved in the nucleation, transforming Me2+ to Me0 (Fig. 2).16
Metal reduction induced by CAL-B was clearly demonstrated by the FTIR and isoelectric point (pI) analyses of the formed nanobiohybrids by the increase of the signal of carboxylic groups (oxidation of hydroxyl group in Ser/Thr) and a decreased value of the pI of the enzyme respectively.16
Finally, the deposition of the continuously formed metal atoms on the previously generated metal nuclei surface leads to nanoparticles growth,27b generating the final metal nanoparticles (Fig. 2).
3. Applications of novel metal nanoparticles biohybrids
3.1 Nanobiohybrids enzymatic and metallic activity application in synthetic cascade processes
One of the main advantages of these nanobiohybrids is their application as multiple catalysts for a chemical cascade reaction.16,21The dual activity (metal nanoparticles and enzyme catalysis in one) of these nanobiohybrids makes them a very powerful tool in modern organic chemistry. This concept has been demonstrated, for example, in a domino reaction, combining the hydrolytic activity of the enzyme and the reducing activity of the metal, to transform p-nitrophenyl propionate to p-aminophenol in water and at room temperature by a heterogeneous nanobiohybrid (Fig. 3).16,20a,21
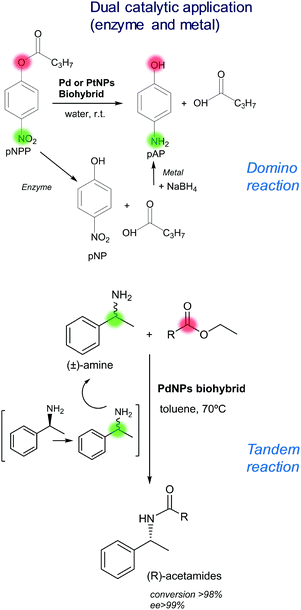 | ||
| Fig. 3 Applications of the novel enzyme–metal nanobiohybrids. Metal reaction (green), enzyme reaction (red). | ||
An additional interesting proof of concept of the potential of these nanocatalysts was to consider the selectivity of the enzyme. In this case, a very useful tandem process was successfully performed by this new bionanohybrid.16 A dynamic kinetic resolution of rac-phenylethylamine was developed by using the CAL-B-PdNPs biohybrid as a catalyst, where an extreme selectivity of the enzyme in the transesterification of R-aryl amine was combined with an excellent racemisation process of the PdNPs of the unreacted S-enantiomer. Thus, the quantitative conversion of enantiopure (R)-benzylamide was possible by a one-pot reaction (Fig. 3).16
These examples open an interesting research line where the high selectivity of the enzyme combined with the broad range of metal catalytic reactions seems to be a perfect arrangement to solve many cascade processes.
3.2 Metal nanoparticles activity application in synthetic reactions
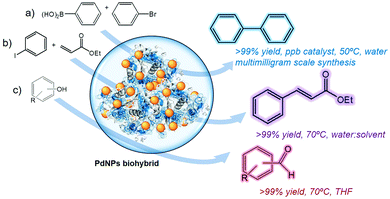
The wide range of reactions that transition metal nanoparticles can catalyze38 provides a very high applicability of these nanobiohybrids in organic chemistry.Some of these novel nanobiohybrids, especially Pd and PtNPs hybrids, were successfully evaluated as catalysts for a set of different chemical reactions,16,18,20a,21 such as reduction, oxidation or C–C bond formation (Fig. 4). It was possible to obtain different kinds of molecules (biaryls, aromatic aldehydes, etc.), interesting as intermediates in the synthesis of pharmaceutical products, polymers, etc. In fact, C–C bond reactions represent one of the most important processes in industry, enabling the preparation of a huge spectrum of molecules, natural products, and polymers.39
It has been found that these heterogeneous materials are excellent catalysts in C–C bond formation in water or in water-co-solvent conditions at moderate temperatures with excellent yields.16,18,32
For example, the CAL-B-PdNPs biohybrid16,32 was extremely active in the Suzuki–Miyaura reaction in water at 50 °C, being an ultra-reactive catalyst (a quantity of ppb was used) to produce biaryls at a multimilligram scale (Fig. 4a).16,32
The Heck reaction represents another well-known methodology of C–C bonding to synthesize biologically active compounds.39 It has been reported that the reaction between aromatic halides and acrylates requires normally hard conditions in terms of solvent and high T to obtain successful yields.39 This has been an excellent example to evaluate the advantages of the catalytic metal nanoparticles by means of biohybrids under relatively moderate conditions. The so-called nanobiohybrids CAL-B-PdNPs and GTL-S193C-p-PdNPs were excellent catalysts in the stereoselective synthesis of trans-ethyl cinnamate from iodobenzene and ethyl acrylate with a >99% conversion at 60–70 °C in an aqueous-solvent medium (Fig. 4b).16,18
The selective oxidation of alcohols to aldehydes has been another interesting reaction tested.21 Different heterogeneous CRL-PdNPs nanobiohybrids were excellent catalysts in the selective oxidation of benzyl alcohol to benzaldehyde as a single product under mild conditions using air as an oxidant (Fig. 4c). Successful results were also obtained in the oxidation of other primary and secondary aromatic alcohols.21 The metal nanoparticles and the heterogeneous nanocatalysts were highly stable under the corresponding experimental conditions in the different synthetic applications used, and were recycled several times without decreasing their catalytic capacity.16,18
3.3 Nanobiohybrids applied as environmental and antimicrobial agents
Along with the excellent synthetic capability exhibited by these novel nanobiohybrids, interesting results of the application of the activity of the metal nanoparticles biohybrids in other important areas such as environmental remediation or action as antimicrobial agents have been recently demonstrated.25,41Actually, one of the main important problems to solve in environmental remediation on a global scale is the contamination of waste and drinking water by organic pollutants.40
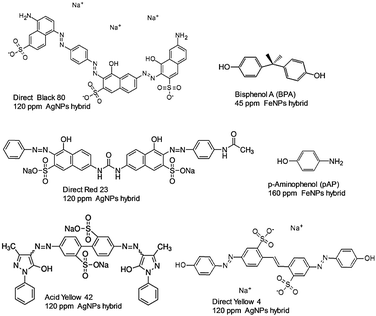
Nanobiohybrids containing AgNPs have been successfully produced for complete degradation of different dyes (Fig. 5).25
The nanobiohybrid containing AgNPs, synthesized by a galactosidase, were a very efficient catalyst for the degradation of different industrial dyes (direct, reductive, and acid dyes), eliminating more than 90% of direct yellow 4, direct black 80, direct red 23, and acid yellow 42, while around 70% was obtained for reduced red and reduced orange (Fig. 5).25
Also, very recently, novel superparamagnetic iron nanoparticles biohybrids were excellent catalysts in the oxidative degradation of p-aminophenol in water (Fig. 5).29
Very promising results have been demonstrated in the decontamination of waste water with very toxic bisphenol-A using metal nanoparticles biohybrids (Fig. 5).24
Another important application of nanobiohybrids has been shown as antimicrobial agents.22a,25,41
The silver nanoparticles-galactosidase biohybrid was also effective as an antibacterial agent inhibiting the growth of Gram negative bacteria (E. coli, P. aeruginosa) and Gram positive bacteria (S. aureus and S. epidermidis), where Gram negative bacteria were more strongly inhibited by these AgNPs.25
Bionanohybrids of AgNPs synthesized using keratinase from Stenotrophomonas maltophilia R13 also showed antimicrobial activity against a variety of pathogenic microorganisms, that is, Gram-negative bacteria (Escherichia coli KCCM40880, Klebisiella pneumoniae ATCC13883, Proteus vulgaris ATCC13315, Salmonella choleraesuis ATCC6994, Salmonella typhimurium KCCM 40253, Serratia marcescens KCCM21204, and Vibrio cholerae KCTC2715), Gram-positive bacteria (Listeria monocytogenes KCCM40307, Staphylococcus aureus ATCC6538), and a yeast (Candida albicans IFO 1385).22a The action mechanism of AgNPs involved structural deformation of cells resulting in membrane leakage and subsequent lysis.
These results may open the door to the future application of new metal nanoparticles-enzyme hybrids in these fields.
4. Conclusion and future remarks
This review highlights developments in a new exciting strategy to synthesize metal nanoparticles, where the use of an enzyme presents a critical role in controlling the size, and generating stable and monodispersed homogeneous nanoparticles. The developed technology allows the synthesis of metal nanoparticles-enzyme biohybrids at multi-milligram scale by simply using an enzyme liquid and water at room temperature without the need for any special equipment. The benefits of this technology such as avoiding the nanoparticles aggregation problems, generating very fancy heterogeneous materials, easy recovery, high degree of recyclability, catalytic performance in organic synthetic chemistry, antimicrobial activity or dual (metallic and enzymatic) activities in the nanobiohybrids have been discussed.Despite the excellent achievements made to date, there are still challenges remaining to be tackled. At this point, some new possible future directions of this technology in terms of design and application could be:
(a) the extension of the technology to other metals. The natural richness of these metals is low so it could be interesting to export this technology to base metals (highly abundant metals, inexpensive such as Fe, Co, Ni, etc.) where different oxidation states exist and where differences on metal species can be obtained.
(b) The extension of the technology to other proteins. The application of high thermal and solvent-stable enzymes obtained by biodiversity or directed evolution techniques would allow highly robust nanobiohybrids to be achieved for applications under harsh conditions.
(c) Extending the metal nanoparticles biohybrid applications to synthetic organic chemistry.
(d) Considering the virtue of these nanobiohybrids to present several activities, extending their applications in cascade processes. At this point, for example, the generation of nanobiohybrids combining two metals with the enzymatic activity to multiple synthesis could be an interesting issue.
(e) For remediation applications, the development of alternative robust metal nanoparticles biohybrids avoiding possible low recyclability or stability.
(f) Apart from the direct catalytic activities of these nanobiohybrids, other functions such as artificial metalloenzyme-like activity (e.g. catalases, and peroxidases) could be studied.
(g) Preparation of metal NPs biohybrids on mesoporous supports suitable for heterogeneous catalysis.
(h) Other new future directions in application of these highly dispersed nanoparticles could be focused in the area of biomedicine by the generation of biosensors for drugs detecting diabetes, fingerprints for forensic processes or energy conversion by designing novel battery electrodes for solar cells.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish Government, the Spanish National Research Council (CSIC) (grant no. CSIC-PIE 201880E011), the Spanish Government (Project AGL2017-84614-C2-2-R), SAMSUNG by GRO PROGRAM 2017, Ramon Areces Foundation and the Ministry of Education, Youth and Sports of the Community of Madrid and the European Social Fund (PEJD-2017PRE/SAL-3762).References
- (a) Y. Hu, J. O. Jensen, W. Zhang, L. N. Cleemann, W. Xing, N. J. Bjerrum and Q. Li, Angew. Chem., Int. Ed., 2014, 53, 3675–3679 CrossRef CAS PubMed; (b) Y.-Z. Chen, Q. Xu, S.-H. Yu and H.-L. Jiang, Small, 2014, 11, 71–76 CrossRef PubMed; (c) Y. Xu, L. Chen, X. Wang, W. Yao and Q. Zhang, Nanoscale, 2015, 7, 10559–10583 RSC; (d) K. L. Luska, A. Bordet, S. Tricard, I. Sinev, W. Grünert, B. Chaudret and W. Leitner, ACS Catal., 2016, 6, 3719–3726 CrossRef CAS.
- (a) P. L. Saldanha, V. Lesnyak and L. Manna, Nano Today, 2017, 12, 46–63 CrossRef CAS; (b) A. B. Soliman, M. H. Hassan, T. N. Huan, A. A. Abugable, W. A. Elmehalmey, S. G. Karakalos, M. Tsotsalas, M. Heinle, M. Elbahri, M. Fontecave and M. H. Alkordi, ACS Catal., 2017, 7, 7847–7854 CrossRef CAS; (c) M. Shviro, S. Polani, R. E. Dunin-Borkowski and D. Zitoun, Adv. Mater. Interfaces, 2018, 5, 1701666 CrossRef.
- Y. Wang, F. Wang, Y. Shen, Q. He and S. Guo, Mater. Horiz., 2018, 5, 184 RSC.
- (a) A. K. Mittal, Y. Chisti and U. C. Banerjee, Biotechnol. Adv., 2013, 31, 346–356 CrossRef CAS PubMed; (b) Z.-U.-R. Mashwani, T. Khan, M. A. Khan and A. Nadhman, Appl. Microbiol. Biotechnol., 2015, 99, 9923–9934 CrossRef CAS PubMed.
- (a) S. R. Chinnadayyala, M. Santhosh, N. K. Singh and P. Goswami, Biosens. Bioelectron., 2015, 69, 155–161 CrossRef CAS PubMed; (b) A. A. Sousa, S. A. Hassan, L. L. Knittel, A. Balbo, M. A. Aronova, P. H. Brown, P. Schuck and R. D. Leapman, Nanoscale, 2016, 8, 6577–6588 RSC.
- P. Vetchinkina, A. E. Loshchinina, I. R. Vodolazov, V. F. Kursky, L. A. Dykman and V. E. Nikitina, Appl. Microbiol. Biotechnol., 2017, 101, 1047–1062 CrossRef PubMed.
- L. Gonzalez-Moragas, L. L. Maurer, V. M. Harms, J. N. Meyer, A. Laromaine and A. Roig, Mater. Horiz., 2017, 4, 719 RSC.
- (a) M. P. Patil and G.-D. Kim, Colloids Surf., B, 2018, 172, 487–495 CrossRef CAS PubMed; (b) S. Ghosh, Appl. Microbiol. Biotechnol., 2018, 102, 7693–7701 CrossRef CAS PubMed; (c) D. Makarovsky, L. Fadeev, B. B. Salam, E. Zelinger, O. Matan, J. Inbar, E. Jurkevitch, M. Gozin and S. Burdman, Appl. Environ. Microbiol., 2018, 84, e02212–e02217 Search PubMed.
- (a) P. Sharma, S. Pant, S. Rai, R. B. Yadav, S. Sharma and V. Dave, Appl. Organomet. Chem., 2018, 32, e4146 CrossRef; (b) M. Baghayeri, B. Mahdavi, Z. Hosseinpor-Mohsen Abadi and S. Farhadi, Appl. Organomet. Chem., 2018, 32, e4057 CrossRef; (c) W. Wang, B. Zhang, Q. Liu, P. Du, W. Liu and Z. He, Environ. Sci.: Nano, 2018, 5, 730–739 RSC.
- X. Zhang, S. Yan, R. D. Tyagi and R. Y. Surampalli, Chemosphere, 2011, 82, 489–494 CrossRef CAS PubMed.
- A. B. Moghaddam, F. Namvar, M. Moniri, P. Md Tahir, S. Azizi and R. Mohamad, Molecules, 2015, 20, 16540–16565 CrossRef CAS PubMed.
- D. Philip, Spectrochim. Acta, Part A, 2009, 15, 374–381 CrossRef PubMed.
- (a) S. K. Das, M. R. Khan, A. K. Guhab and N. Naskar, Green Chem., 2013, 15, 2548–2557 RSC; (b) S. K. Das, T. Parandhaman, N. Pentela, A. K. M. M. Islam, A. B. Mandal and M. Mukherjee, J. Phys. Chem. C, 2014, 118, 24623–24632 CrossRef CAS; (c) M. Gholami-Shabania, M. Shams-Ghahfarokhic, Z. Gholami-Shabanid, A. Akbarzadehb, G. Riazie, S. Ajdarif, A. Amanig and M. Razzaghi-Abyaneh, Process Biochem., 2015, 50, 1076–1085 CrossRef; (d) S. R. Chinnadayyala, M. Santhosh, N. K. Singh and P. Goswami, Biosens. Bioelectron., 2015, 69, 155–161 CrossRef CAS PubMed.
- (a) Z. Zhou, G. J. Bedwell, R. Li, P. E. Prevelige and A. Gupta, Sci. Rep., 2014, 4, 3832 CrossRef PubMed; (b) L. Zhang and F. Han, Nanotechnology, 2018, 29, 165702 CrossRef PubMed; (c) Y. Li, K. Song, Y. Cao, C. Peng and G. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 26039 CrossRef CAS PubMed; (d) A. Soleilhac, F. Bertorelle and R. Antoine, Spectrochim. Acta, Part A, 2018, 193, 283–288 CrossRef CAS PubMed; (e) J. Sheng, L. Wang, Y. Han, W. Chen, H. Liu, M. Zhang, L. Deng and Y.-N. Liu, Small, 2018, 14, art. no. 1702529 CrossRef PubMed; (f) S. M. Lystvet, S. Volden, G. Singh, I. M. Rundgren, H. Wen, Ø. Halskauc and W. R. Glomm, J. Phys. Chem. C, 2013, 117, 2230 CrossRef CAS; (g) S. M. Lystvet, S. Volden, G. Singh, M. Yasuda, Ø. Halskauc and W. R. Glomm, RSC Adv., 2013, 3, 482 RSC.
- F. Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Chem. Rev., 2018, 118, 142–231 CrossRef CAS PubMed.
- M. Filice, M. Marciello, M. P. Morales and J. M. Palomo, Chem. Commun., 2013, 49, 6876–6878 RSC.
- V. Gotor-Fernández, E. Busto and V. Gotor, Adv. Synth. Catal., 2006, 348, 797–812 CrossRef.
- D. Lopez-Tejedor, B. de las Rivas and J. M. Palomo, Molecules, 2018, 23, 2358 CrossRef PubMed.
- T.-T. Chen, J.-T. Yi, Y.-Y. Zhao and X. Chu, J. Am. Chem. Soc., 2018, 140, 9912–9920 CrossRef CAS PubMed.
- (a) B. H. San, S. Ravichandran, K. Park, V. K. Subramani and K. K. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 30058–30065 CrossRef CAS PubMed; (b) C.-J. Yu, T. H. Chen, J.-Y. Jiang and W.-L. Tseng, Nanoscale, 2014, 6, 9618–9624 RSC.
- P. O’Brien, D. Lopez-Tejedor, R. Benavente and J. M. Palomo, ChemCatChem, 2018, 10, 5006–5013 Search PubMed.
- (a) E.-Y. Jang, Y.-J. Son, S.-Y. Park, J.-Y. Yoo, Y.-N. Cho, S.-Y. Jeong, S. Liu and H.-J. Son, Bioprocess Biosyst. Eng., 2018, 41, 381–393 CrossRef CAS PubMed; (b) H. Cai and P. Yao, Nanoscale, 2013, 5, 2892 RSC.
- (a) S. A. Wadhwania, U. U. Shedbalkarb, R. Singha and B. A. Chopade, Enzyme Microb. Technol., 2018, 111, 81–86 CrossRef PubMed; (b) B. Sharma, S. Mandani and T. K. Sarma, J. Mater. Chem. B, 2014, 2, 4072 RSC.
- R. Benavente, D. Lopez-Tejedor and J. M. Palomo, Chem. Commun., 2018, 54, 6256–6259 RSC.
- F. Ahmeda, S. Qayyumb and Q. Husain, Mater. Sci. Eng., C, 2018, 91, 570–578 CrossRef PubMed.
- Z. Li, Y. Ding, X. Wu, J. Ge, P. Ouyangb and Z. Liu, RSC Adv., 2016, 6, 20772 RSC.
- (a) B. Maity, S. Abe and T. Ueno, Nat. Commun., 2017, 8, 14820 CrossRef CAS PubMed; (b) B. Ingham, T. H. Lim, C. J. Dotzler, A. Henning, M. F. Toney and R. D. Tilley, Chem. Mater., 2011, 23, 3312–3317 CrossRef CAS.
- S. Qiao, R. Wang, T. Yan, X. Li, L. Zhao, X. Zhang, X. Fan, T. Wang, Y. Liu, C. Hou, Q. Luo, J. Xu and J. Liu, Part. Part. Syst. Charact., 2018, 35, 1700436 CrossRef.
- R. Benavente, D. Lopez-Tejedor, C. Perez-Rizquez and J. M. Palomo, Molecules, 2018, 23, 2166 CrossRef PubMed.
- J. N. Araújo, A. Tofanelloa, V. M. da Silvaa, J. A. P. Satoa, F. M. Squinab, I. L. Nantesa and W. Garcia, Int. J. Biol. Macromol., 2017, 102, 84–91 CrossRef PubMed.
- T. Görbe, K. P. J. Gustafson, O. Verho, G. Kervefors, H. Zheng, X. Zou, E. V. Johnston and J.-E. Bäckvall, ACS Catal., 2013, 7, 1601–1605 CrossRef.
- T. Cuenca, M. Filice and J. M. Palomo, Enzyme Microb. Technol., 2016, 95, 242–247 CrossRef CAS PubMed.
- Artificial Metalloenzymes and MetalloDNAzymes in Catalysis: From Design to Applications. ISBN: 978-3-527-80409-2. ed. Montserrat Diéguez, Jan-E. Bäckvall, Oscar Pàmies. 2018, p. 1200.
- M. Filice, O. Romero, A. Aires, J. M. Guisan, A. Rumbero and J. M. Palomo, Adv. Synth. Catal., 2015, 357, 2687–2696 CrossRef CAS.
- (a) M. Filice and J. M. Palomo, ACS Catal., 2014, 4, 1588–1598 CrossRef CAS; (b) L. Han, H. Zhang, D. Chen and F. Li, Adv. Funct. Mater., 2018, 28, art. no. 1800018 CrossRef.
- S. Ding, A. A. Cargill, I. L. Medintz and J. C. Claussen, Curr. Opin. Biotechnol., 2015, 34, 242–250 CrossRef CAS PubMed.
- Y. N. Tan, J. Y. Lee and D. I. C. Wang, J. Am. Chem. Soc., 2010, 132, 5677–5686 CrossRef CAS PubMed.
- (a) G. Dodekatos, S. Schünemann and H. Tüysüz, ACS Catal., 2018, 8, 6301–6333 CrossRef CAS; (b) C. Hanske, M. N. Sanz-Ortiz and L. M. Liz-Marzán, Adv. Mater., 2018, 30, 1707003 CrossRef PubMed; (c) G. Li and Z. Tang, Nanoscale, 2014, 6, 3995–4011 RSC.
- P. P. Mpungose, Z. P. Vundla, G. E. M. Maguire and H. B. Friedrich, Molecules, 2018, 23, pii: E1676 CrossRef PubMed.
- J. C. G. Sousa, A. R. Ribeiro, M. O. Barbosa, M. Fernando, R. Pereira and A. M. T. Silva, J. Hazard. Mater., 2018, 344, 146–162 CrossRef CAS PubMed.
- L.-Y. Tao, J.-S. Gong, C. Su, M. Jiang, H. Li, H. Li, Z.-M. Lu, Z.-H. Xu and J.-S. Shi, ACS Biomater. Sci. Eng., 2018, 4, 1307–1315 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |