Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Turning on ROP activity in a bimetallic Co/Zn complex supported by a [2+2] Schiff-base macrocycle†
Kuiyuan
Wang
,
Timothy J.
Prior
and
Carl
Redshaw
 *
*
Department of Chemistry & Biochemistry, University of Hull, Hull, HU6 7RX, UK. E-mail: c.redshaw@hull.ac.uk; Tel: +44 1482 465219
First published on 16th August 2019
Abstract
Homo-dinuclear Co and Zn complexes derived from the macrocycle LH2, {[2-(OH)-5-(R)-C6H2-1,3-(CH)2][CH2CH2(2-C6H4N)2]}2 (R = Me, tBu), revealed near inactivity for the ring opening polymerization (ROP) of the cyclic esters δ-valerolactone (δ-VL) and ε-caprolactone (ε-CL). By contrast, the hetero-bimetallic complexes [LCo(NCMe)(μ-Br)ZnBr]·nMeCN (n = 3 or 3.25) were found to be efficient catalysts for the ROP of ε-CL and δ-VL.
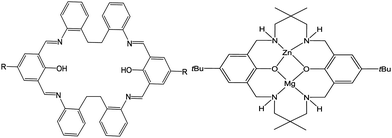
The production of aliphatic polyesters has become a very topical area given their biodegradability and biocompatibility,1 and given the current global issues with plastic pollution, this is likely to remain the case for the foreseeable future.2 One viable route to such polymers is via the metal-catalyzed ring opening polymerization (ROP) of cyclic esters.3 The choice of metal catalyst is influenced by a number factors, such as price, toxicity, activity and control. Frameworks that are capable of simultaneously binding multiple metal centres continue to be of great interest in various fields of catalysis, and this stems from the possible presence of favourable cooperative effects.4 We have become interested in the use of Schiff-base macrocycles given that they possess multiple binding sites,5 and our studies have been focusing on the simplest members of this family, the so-called Robson type macrocycles, derived from the [2+2] condensation of a diamine/dianiline with a dialdehyde.6 In terms of catalysis (ROP of ε-CL), we observed beneficial cooperative effects when alkylaluminium centres are bound in a specific way to a Schiff-base macrocycle (LH2, Scheme 1, left) derived from the dianiline [(CH2CH2)(2-C6H4NH2)2], whereas the presence of aluminoxane type (Al–O–Al) bonding in such a system proved detrimental.7 Furthermore, we noted that manganese complexes of such macrocycles were far less active with conversions for the ROP of ε-CL <15%.8 It is noteworthy that the structural chemistry of this particular macrocycle is underexplored, indeed a search of the CSD revealed no hits,9 other than our previously mentioned chemistry.7,8 We have selected the metals cobalt and zinc given their relatively low cost and biocompatibility. Further, we note that the use of Zn complexes for the ROP of ε-CL is well established,3 whilst reports on ROP using Co species is scant.10
Recent work by Williams et al. has shown how the use of hetero-dinuclear complexes can result in enhanced catalytic performance.11a In particular, it was reported that an in situ generated catalyst comprising the hetero-dinuclear Zn–Mg complex bearing a diphenolate tetraamine macrocycle (Scheme 1, right) used in conjunction with its homo-dinuclear Zn–Zn and Mg–Mg counterparts performed better for the copolymerization of CO2 with epoxides than did the homo-dinuclear complexes alone. Given the very similar solubility properties of the three species, it was not possible to crystallize selectively the Mg–Zn hetero-dinuclear complex. However, subsequent studies by the same group have shown that it is possible to access such species via mono-metalation, followed by the addition of the second metal,11b and the molecular structures of a number of mixed-metal species were reported.11c–e Interestingly, Williams et al. also observed that for ROP of ε-CL and r-LA, a mixed Ti/Zn system displayed moderate/high activity whilst the mono-Ti system was inactive.11c We also note that cooperative effects have also been observed for a homo-dinuclear zinc complex in lactide polymerization.12
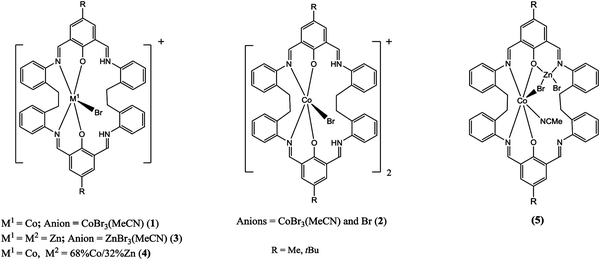
Given this, we have re-focused our efforts on the [2+2] Schiff-base systems and have extended our studies in an attempt to access mixed-metal systems. Herein, we report that homo-dinuclear cobalt and zinc complexes bearing the macrocycle L (1–3, Scheme 2) are readily accessible. Furthermore, on reacting 1 with Br2Zn a complex 4 related to 1 was formed differing only in the composition (partial occupancy Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 68
Zn = 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32) of the anion, whereas with Et2Zn, it proved possible to isolate and structurally characterize the mixed-metal Co–Zn complexes 5 (Scheme 2 right). Interestingly, the homo-dinuclear species 1 and 3 (R = Me and tBu) were inactive when screened for the ROP of ε-CL (and virtually inactive for ε-VL), whereas the mixed-metal complexes 5Me and 5tBu were efficient catalysts when screened under the same conditions (130 °C, 24 h). We note that poly(ε-caprolactone), PCL and PVL are favoured polymers given both their biodegradability, and are considered as potential environmentally friendly commodity plastics.13 Beyond ROP, Co–Zn species are of interest in gas sensors, semiconductors, dyes and pigments, as well as in other catalytic processes.14
32) of the anion, whereas with Et2Zn, it proved possible to isolate and structurally characterize the mixed-metal Co–Zn complexes 5 (Scheme 2 right). Interestingly, the homo-dinuclear species 1 and 3 (R = Me and tBu) were inactive when screened for the ROP of ε-CL (and virtually inactive for ε-VL), whereas the mixed-metal complexes 5Me and 5tBu were efficient catalysts when screened under the same conditions (130 °C, 24 h). We note that poly(ε-caprolactone), PCL and PVL are favoured polymers given both their biodegradability, and are considered as potential environmentally friendly commodity plastics.13 Beyond ROP, Co–Zn species are of interest in gas sensors, semiconductors, dyes and pigments, as well as in other catalytic processes.14
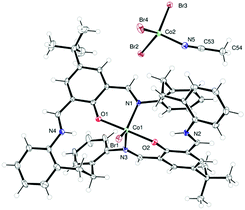
Reaction of the [2+2] Schiff-base macrocycle {[2-(OH)-5-(R)-C6H2-1,3-(CH)2][CH2CH2(2-C6H4N)2]}2 (R = Me, tBu) with 2.1 equivalents of CoBr2 afforded, following work-up, green prisms on recrystallization from a saturated solution of acetonitrile at 0 °C in about 70% yield. The molecular structure of 1tBu·0.5MeCN is shown in Fig. 1, with selected bond lengths and angles given in the caption; for 1Me·4MeCN see ESI,† Fig. S1. The complex is a salt of formula [CoBrLtBu][CoBr3(NCMe)]·0.5MeCN (1tBu·0.5MeCN). In the cation, the cobalt centre is five-coordinate with a trigonal bipyramidal geometry; the apical sites are occupied by O atoms of phenolates, with the bromide and two N atoms in the equatorial sites. Concentration of the mother-liquor afforded more of 1tBu·0.5MeCN plus small amounts of an orange/red product, identified as [CoBrLtBu]2[CoBr3(NCMe)][Br]·4.5MeCN (2tBu·4.5MeCN), the structure of which is similar to that observed for 1tBu·0.5MeCN, differing mainly in the composition of the anions, namely Br− and CoBr3(NCMe)−; the molecular structure is shown in Fig. S2 (ESI†). Similar interaction of LH2 with 2.1 equivalents of ZnBr2 afforded, following work-up, yellow prisms of [ZnLtBuBr][ZnBr3(NCMe)]·MeCN (3tBu·MeCN) in good yield. Use of excess ZnBr2 (>4 equivalents) also afforded 3tBu·MeCN as the only crystalline product. Again, the structure (ESI,† Fig. S4) is very reminiscent of 1tBu·0.5MeCN, with the zinc adopting a trigonal bipyramidal geometry in the cation; for the structure of 3Me·MeCN, see Fig. S3 (ESI†). Having isolated and characterized these homo-dinuclear products, we then targeted the formation of a mixed-metal system. Complex 1tBu was reacted with ZnBr2 to afford a yellow product, however the structure, namely [CoBrLtBu][Co0.68Zn0.32Br3(NCMe)]·0.25MeCN (4tBu·0.25MeCN), turned out to be very similar to 1 differing only in the composition of the anion, the latter having cobalt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc occupancy 68.4
zinc occupancy 68.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
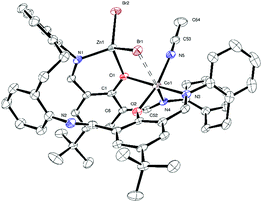
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.6(17) (ESI,† Fig. S5). We note that the structures of both 3Me/tBu and 4tBu are analogous to the equivalent manganese complex (see CUVYID in CCDC).7c Given this disappointing result, both 1Me and 1tBu were treated with Et2Zn (one equivalent) and on work-up (acetonitrile), brown/red products were isolated in good yield. Single crystals were grown from acetonitrile on prolonged standing (3 days) at ambient temperature. The molecular structure of 5tBu·3.25MeCN is shown in Fig. 2, with bond lengths/angles given in the caption; for 5Me·3MeCN see Fig. S6, ESI.† In 5tBu·3.25MeCN, the macrocycle binds two metals (Co and Zn) in an ordered way. The Co2+ is bound in distorted octahedral geometry by two cis phenolates and two cis imines of the macrocycle, a rather distant bromide (Co(1)–Br(1) 2.9383(5) Å), and a single molecule of acetonitrile. The Zn ion is in close proximity to the Co ion: the bromide and one of the phenolates each coordinate to the Zn. The tetrahedral coordination about the Zn is completed by an imine and a mono-dentate bromide. One might describe the octahedron of Co(1) and the tetrahedron around Zn(1) as sharing a common edge (O(1) and Br(1)). The two metals are separated at a distance 3.2399(5) Å. The magnetic moments of 5 (R = Me, tBu) are in the range 5.1–5.3 μB consistent with high spin Co(II) complexes,15 whilst the 1H NMR spectra are broad and spread over a large window (+140 to −40 ppm). The cobalt and zinc complexes 1, 3 and 5 (R = Me, tBu) have been screened for their ability to ring open polymerize δ-VL and ε-CL; runs were conducted in the presence of benzyl alcohol (BnOH). For complexes 1 and 3, a variety of conditions were used in the attempted ROP of δ-VL including temperatures in the range 40 to 130 °C, differing ratios of [δ-VL]
32.6(17) (ESI,† Fig. S5). We note that the structures of both 3Me/tBu and 4tBu are analogous to the equivalent manganese complex (see CUVYID in CCDC).7c Given this disappointing result, both 1Me and 1tBu were treated with Et2Zn (one equivalent) and on work-up (acetonitrile), brown/red products were isolated in good yield. Single crystals were grown from acetonitrile on prolonged standing (3 days) at ambient temperature. The molecular structure of 5tBu·3.25MeCN is shown in Fig. 2, with bond lengths/angles given in the caption; for 5Me·3MeCN see Fig. S6, ESI.† In 5tBu·3.25MeCN, the macrocycle binds two metals (Co and Zn) in an ordered way. The Co2+ is bound in distorted octahedral geometry by two cis phenolates and two cis imines of the macrocycle, a rather distant bromide (Co(1)–Br(1) 2.9383(5) Å), and a single molecule of acetonitrile. The Zn ion is in close proximity to the Co ion: the bromide and one of the phenolates each coordinate to the Zn. The tetrahedral coordination about the Zn is completed by an imine and a mono-dentate bromide. One might describe the octahedron of Co(1) and the tetrahedron around Zn(1) as sharing a common edge (O(1) and Br(1)). The two metals are separated at a distance 3.2399(5) Å. The magnetic moments of 5 (R = Me, tBu) are in the range 5.1–5.3 μB consistent with high spin Co(II) complexes,15 whilst the 1H NMR spectra are broad and spread over a large window (+140 to −40 ppm). The cobalt and zinc complexes 1, 3 and 5 (R = Me, tBu) have been screened for their ability to ring open polymerize δ-VL and ε-CL; runs were conducted in the presence of benzyl alcohol (BnOH). For complexes 1 and 3, a variety of conditions were used in the attempted ROP of δ-VL including temperatures in the range 40 to 130 °C, differing ratios of [δ-VL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cat]
[Cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] and over different times, however on each occasion there was no sign of polymer upon work-up. However, for the mixed-metal system 5, activity was observed when the temperature reached 130 °C, in the presence of one equivalent of BnOH (at this temperature, no activity was observed for 1 and 3 – see Fig. 3 for the relative rates of the tBu derivatives). Data for runs is given in Table 1 (see also Fig. S19–S24, ESI†) and reveals that 5tBu out-performs 5Me, which is thought to be due to the increased solubility of the former in the reaction solvent. In these mixed-metal systems, the Co to Zn distance may favour coordination of a monomer to both metal centres, and then as in the ROP of propylene oxide,16 one metal acts a as Lewis acid with the other using its M-OR function to attack the carbonyl group. Highest conversions were achieved with ratios between 100
[BnOH] and over different times, however on each occasion there was no sign of polymer upon work-up. However, for the mixed-metal system 5, activity was observed when the temperature reached 130 °C, in the presence of one equivalent of BnOH (at this temperature, no activity was observed for 1 and 3 – see Fig. 3 for the relative rates of the tBu derivatives). Data for runs is given in Table 1 (see also Fig. S19–S24, ESI†) and reveals that 5tBu out-performs 5Me, which is thought to be due to the increased solubility of the former in the reaction solvent. In these mixed-metal systems, the Co to Zn distance may favour coordination of a monomer to both metal centres, and then as in the ROP of propylene oxide,16 one metal acts a as Lewis acid with the other using its M-OR function to attack the carbonyl group. Highest conversions were achieved with ratios between 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 500
1 and 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 130 °C, with all the runs showing good control with PDIs in range 1.15–1.30; above and below this temperature only trace polymer was observed. Use of either 12 h (run 6) or no solvent (i.e. a melt, run 7) was less controlled. Analysis of the polymer indicated the presence of benzyloxy and hydroxyl end groups (Fig. S22, ESI†). There was evidence of transesterification and all observed Mn values were significantly lower than the calculated values (see Fig. S21, ESI†). In the case of ε-CL, the situation was more pronounced with no conversion evident by 1H NMR spectroscopy for catalyst systems 1 and 3. By contrast, systems using 5Me and 5tBu were efficient catalysts at 130 °C over 24 h, with similar conversion results observed using the ratios between 50
1 at 130 °C, with all the runs showing good control with PDIs in range 1.15–1.30; above and below this temperature only trace polymer was observed. Use of either 12 h (run 6) or no solvent (i.e. a melt, run 7) was less controlled. Analysis of the polymer indicated the presence of benzyloxy and hydroxyl end groups (Fig. S22, ESI†). There was evidence of transesterification and all observed Mn values were significantly lower than the calculated values (see Fig. S21, ESI†). In the case of ε-CL, the situation was more pronounced with no conversion evident by 1H NMR spectroscopy for catalyst systems 1 and 3. By contrast, systems using 5Me and 5tBu were efficient catalysts at 130 °C over 24 h, with similar conversion results observed using the ratios between 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 500
1 and 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; above and below this temperature only trace polymer was observed. As for the δ-VL runs, good control was observed, except for when using either 12 h or a melt. From a kinetic study, it was observed that the polymerization rate exhibited near first order dependence of the CL concentration at 130 °C. The rate of polymerization for ε-CL (Kobs = 2.12 × 10−3 (h−1) for 5tBu) was less than half that for δ-VL (Kobs = 6.10 × 10−3 (h−1) for 5tBu), which is consistent with other reports.17 The observed molecular weights were lower than the calculated values, suggesting the presence of a chain transfer agent (H2O or BnOH). This was also evident in the MALDI-TOF mass spectra, were a number of families of peaks were observed separated by 114 mass units (Fig. S26 and S27, ESI†); the highest were typically about 7000. For example, in the case of 5tBu (run 4, Table 2), peaks in the spectrum
can be assigned to 4926, 5039, and 5154 using the formula C6H6–CH2O[O(CH2)4CO]nOH for n = 42, 43 and 44 respectively. The 1H NMR spectra of the PCL (e.g. Fig. S28, ESI†) revealed the presence of benzyloxy and OH end groups. It also proved possible to ROP r-LA using the mixed-metal systems, albeit in much poorer yields. Moreover, co-polymerization of ε-VL and ε-CL was possible as was ε-CL with r-LA (see Table S1 and Fig. S33–S43, ESI† for preliminary results).
1; above and below this temperature only trace polymer was observed. As for the δ-VL runs, good control was observed, except for when using either 12 h or a melt. From a kinetic study, it was observed that the polymerization rate exhibited near first order dependence of the CL concentration at 130 °C. The rate of polymerization for ε-CL (Kobs = 2.12 × 10−3 (h−1) for 5tBu) was less than half that for δ-VL (Kobs = 6.10 × 10−3 (h−1) for 5tBu), which is consistent with other reports.17 The observed molecular weights were lower than the calculated values, suggesting the presence of a chain transfer agent (H2O or BnOH). This was also evident in the MALDI-TOF mass spectra, were a number of families of peaks were observed separated by 114 mass units (Fig. S26 and S27, ESI†); the highest were typically about 7000. For example, in the case of 5tBu (run 4, Table 2), peaks in the spectrum
can be assigned to 4926, 5039, and 5154 using the formula C6H6–CH2O[O(CH2)4CO]nOH for n = 42, 43 and 44 respectively. The 1H NMR spectra of the PCL (e.g. Fig. S28, ESI†) revealed the presence of benzyloxy and OH end groups. It also proved possible to ROP r-LA using the mixed-metal systems, albeit in much poorer yields. Moreover, co-polymerization of ε-VL and ε-CL was possible as was ε-CL with r-LA (see Table S1 and Fig. S33–S43, ESI† for preliminary results).
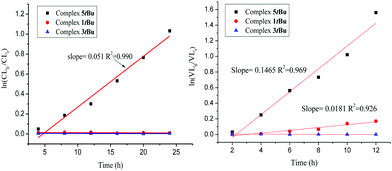 | ||
Fig. 3 Plot of ln[VL]0/[VL]tvs. time and ln[CL]0/[CL]tvs. time using complex 1tBu, 3tBu and 5tBu ([monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cat] [Cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 500 [BnOH] = 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 130 °C. 1); 130 °C. | ||
| Run | Cat. | [δ-VL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cat] [Cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] [BnOH] |
T (°C) | t (h) | Conv.a (%) |
M
n × 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
nCalcd × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
PDId |
|---|---|---|---|---|---|---|---|---|
| a By 1H NMR spectroscopic analysis. b M n values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factors (0.58 for poly(δ-VL)). c (F.W.[M]/[BnOH])(conversion). d Polydispersity index (Mw/Mn) were determined by GPC. | ||||||||
| 1 | 5tBu | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 88.7 | 1.35 | 0.45 | 1.16 |
| 2 | 5tBu | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 92.8 | 2.14 | 0.93 | 1.15 |
| 3 | 5tBu | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 98.7 | 5.19 | 2.46 | 1.22 |
| 4 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 99.4 | 6.89 | 4.97 | 1.23 |
| 5 | 5tBu | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 77.9 | 5.33 | 7.79 | 1.43 |
| 6 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 12 | 92.7 | 0.45 | 4.64 | 1.53 |
| 7 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (melt) 1 (melt) |
130 | 24 | 96.2 | 7.11 | 4.82 | 1.79 |
| 8 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 24 | 76.1 | 0.76 | 3.80 | 1.19 |
| 9 | 5Me | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 97.0 | 2.68 | 2.43 | 1.30 |
| 10 | 5Me | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 91.4 | 3.35 | 4.57 | 1.15 |
| 11 | 1tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 38.8 | 0.45 | 1.94 | 1.42 |
| 12 | 3tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 4.4 | — | 0.22 | — |
| Run | Cat. | [ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cat] [Cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) |
M
n × 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
nCalcd × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
PDId |
|---|---|---|---|---|---|---|---|---|
| a By 1H NMR spectroscopic analysis. b M n values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factors (0.56 for poly(ε-CL)). c (F.W.[M]/[BnOH])(conversion). d Polydispersity index (Mw/Mn) were determined by GPC. | ||||||||
| 1 | 5tBu | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 99.3 | 1.14 | 0.57 | 1.14 |
| 2 | 5tBu | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 99.6 | 2.65 | 1.01 | 1.13 |
| 3 | 5tBu | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 99.6 | 4.10 | 2.86 | 1.13 |
| 4 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 99.8 | 6.56 | 5.05 | 1.34 |
| 5 | 5tBu | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 88.7 | 3.61 | 8.87 | 1.12 |
| 6 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 12 | 48.0 | 2.28 | 2.74 | 1.80 |
| 7 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 71.5 | 3.48 | 1.78 | 1.15 |
| 8 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (melt) 1 (melt) |
130 | 24 | 98.2 | 7.87 | 4.98 | 1.66 |
| 9 | 5tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 24 | 72.7 | — | 3.63 | — |
| 10 | 5Me | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 36 | 97.6 | 4.83 | 2.44 | 1.34 |
| 11 | 5Me | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 36 | 99.2 | 5.16 | 4.96 | 1.96 |
| 12 | 1tBu | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | — | — | — | — |
| 13 | 1Me | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | — | — | — | — |
In conclusion, we have isolated and structurally characterized homo-dinuclear cobalt and zinc complexes as well as rare examples of mixed-metal (Co/Zn) complexes of a [2+2] macrocyclic Schiff-base. Screening of these complexes for the ROP of the cyclic esters δ-valerolactone (δ-VL) and ε-caprolactone (ε-CL) revealed that whilst the homo-dinuclear complexes are either virtually inactive (δ-VL) or inactive (ε-CL), the mixed-metal systems are efficient catalysts at 130 °C, suggestive that the metals are able to ‘turn each other on’.
We thank the China Scholarship Council (CSC) for a PhD Scholarship with KW. CR thanks the EPSRC (grant EP/S025537/1) for financial support. We thank the EPSRC National Crystallography Service for data collection and the Physical Sciences Data-Science Service for access to the CCDC.
Conflicts of interest
There are no conflicts to declare.References
- A. C. Albertsson and I. K. Varma, Biomacromolecules, 2003, 4, 1466–1486 CrossRef CAS PubMed.
- P. Villarrubia-Gómez, S. E. Cornell and J. Fabres, Marine Policy, 2018, 96, 213–220 CrossRef.
- For reviews, see (a) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef CAS PubMed; (b) M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC; (c) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165–173 RSC; (d) A. Arbaoui and C. Redshaw, Polym. Chem., 2010, 1, 801–826 RSC; (e) Y. Sarazin and J.-F. Carpentier, Chem. Rev., 2015, 115, 3564–3614 CrossRef CAS PubMed and references therein.
- See for example, (a) S. K. Mandal and H. W. Roesky, Acc. Chem. Res., 2010, 43, 248–259 CrossRef CAS PubMed; (b) M. Delferro and T. J. Marks, Chem. Rev., 2011, 111, 2450–2485 CrossRef CAS PubMed; (c) J. P. McInnis, M. Delferro and T. J. Marks, Acc. Chem. Res., 2014, 47, 2545–2557 CrossRef CAS PubMed; (d) I. Bratko and M. GÓmez, Dalton Trans., 2013, 42, 10664–10681 RSC.
- (a) S. Brooker, Coord. Chem. Rev., 2001, 222, 33–56 CrossRef CAS; (b) W. Radecka-Paryzek, V. Patroniak and J. Lisowski, Coord. Chem. Rev., 2005, 249, 2156–2175 CrossRef CAS; (c) C. Redshaw, Dalton Trans., 2016, 45, 9018–9030 RSC; (d) C. Redshaw, Catalysts, 2017, 7, 165 CrossRef.
- (a) N. H. Pilkington and R. Robson, Aust. J. Chem., 1970, 23, 2225–2236 CAS; (b) M. Bell, A. J. Edwards, B. F. Hoskins, E. H. Kachab and R. Robson, J. Am. Chem. Soc., 1989, 111, 3603–3610 CrossRef CAS.
- (a) A. Arbaoui, C. Redshaw and D. L. Hughes, Chem. Commun., 2008, 4717 RSC; (b) A. Arbaoui, C. Redshaw and D. L. Hughes, Supramol. Chem., 2009, 21, 35 CrossRef CAS; (c) W. Yang, K.-Q. Zhao, B.-Q. Wang, C. Redshaw, M. R. J. Elsegood, J.-L. Zhao and T. Yamato, Dalton Trans., 2016, 45, 11990–12005 RSC.
- W. Yang, K.-Q. Zhao, B.-Q. Wang, C. Redshaw, M. R. J. Elsegood, J.-L. Zhao and T. Yamato, Dalton Trans., 2016, 45, 226–236 RSC.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef PubMed.
- See for example, J. Zhang, B. Wang, L. Wang, J. Sun, Y. Zhang, Z. Cao and Z. Wu, Appl. Organomet. Chem., 2018, 32e4077 Search PubMed and references therein.
- (a) P. K. Saini, C. Romain and C. K. Williams, Chem. Commun., 2014, 50, 4164–4167 RSC; (b) J. F. Garden, P. K. Saini and C. K. Williams, J. Am. Chem. Soc., 2015, 137, 15078–15081 CrossRef CAS PubMed; (c) J. F. Garden, A. J. P. White and C. K. Williams, Dalton Trans., 2017, 46, 2532–2541 RSC; (d) A. C. Deacy, C. B. Durr, J. F. Garden, A. J. P. White and C. K. Williams, Inorg. Chem., 2018, 57, 15575–15583 CrossRef CAS PubMed; (e) G. Trott, J. F. Garden and C. K. Williams, Chem. Sci., 2019, 10, 4618–4627 RSC.
- A. Thevenon, C. Romain, M. S. Bennington, A. J. P. White, H. J. Davidson, S. Brooker and C. K. Williams, Angew. Chem., Int. Ed., 2016, 55, 8680–8685 CrossRef CAS PubMed.
- See for example, (a) M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS; (b) Y. F. Al-Khafaji and F. H. Hussein in Green and Sustainable Advanced Materials: Processing and Characterization, ed. S. Ahmed and C. M. Hussain, Wiley, 2018, ch. 6 Search PubMed.
- (a) T. Baird, K. C. Campbell, P. J. Holliman, R. W. Hoyle, D. Stirling, B. P. Williams and M. Morris, J. Mater. Chem., 1997, 7, 319–330 RSC; (b) X. Niu, W. Du and W. Du, Sens. Actuators, B, 2004, 99, 405–409 CrossRef CAS; (c) N. H. Perry, T. O. Mason, C. Ma, A. Navrotsky, S. A. Kondrat, T. E. Davies, D. J. Morgan, D. I. Enache, G. B. Combes, S. H. Taylor, J. K. Bartley and G. J. Hutchings, Catal. Sci. Technol., 2014, 4, 1970–1978 RSC.
- (a) R. W. Handel, H. Willms, G. B. Jameson, K. J. Berry, B. Moubaraki, K. S. Murray and S. Brooker, Eur. J. Inorg. Chem., 2010, 3317–3327 CrossRef CAS; (b) A. A. Abou-Hussein and W. Linert, Spectrochim. Acta, Part A, 2015, 141, 223–232 CrossRef CAS PubMed.
- (a) B. Antelmann, M. H. Chisholm, S. S. Iyer, J. C. Huffman, D. Navarro-Llobet, M. Pagel, W. J. Simonsick and W. Zhong, Macromolecules, 2001, 34, 3159–3175 CrossRef CAS; (b) W. Braune and J. Okuda, Angew. Chem., Int. Ed., 2003, 42, 64–68 CrossRef CAS.
- K. Makiguchi, T. Satoh and T. Kakuchi, Macromolecules, 2011, 44, 1999–2005 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details for complex synthesis and ROP studies. CCDC 1829365–1829367 (1tBu·0.5MeCN, 2tBu·4.5MeCN, and 5tBu·3.25MeCN), 1849421 (4tBu·0.25MeCN) and 1921189–1921192. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc04494a |
| This journal is © The Royal Society of Chemistry 2019 |