Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Locally pH controlled and directed growth of supramolecular gel microshapes using electrocatalytic nanoparticles†
Vasudevan Lakshminarayanan‡
 a,
Lukasz Poltorak
a,
Lukasz Poltorak *ab,
Duco Bosmaa,
Ernst J. R. Sudhölter
*ab,
Duco Bosmaa,
Ernst J. R. Sudhölter a,
Jan H. van Esch
a,
Jan H. van Esch a and
Eduardo Mendes
a and
Eduardo Mendes *a
*a
aDelft University of Technology, Department of Chemical Engineering, Van der Maasweg 9, 2629 HZ Delft, The Netherlands. E-mail: l.poltorak@tudelft.nl; e.mendes@tudelft.nl
bUniversity of Lodz, Faculty of Chemistry, Department of Inorganic and Analytical Chemistry, Electroanalysis and Electrochemistry Group, Tamka 12, 91-403 Lodz, Poland. E-mail: Lukasz.poltorak@chemia.uni.lodz.pl
First published on 2nd July 2019
Abstract
Controlled localization of platinum nanoparticles (Pt NPs) at a solid support assisted by a polarized liquid–liquid interface is reported. Electrocatalytic water oxidation resulted in local pH modulation followed by the directed self-assembly of a dibenzoyl-L-cystine hydrogelator forming a structured hydrogel retaining the shape of the Pt NP deposit.
Supramolecular hydrogels have been reported to find applications in the fields of cell culture1 and drug delivery systems2 among many others. The ability to exert spatial control over the formation of these materials is therefore of high value. Directed self-assembly of supramolecular hydrogelators composed of low molecular weight gelators has seen rapid progress in recent years. With the ability to control supramolecular gel formation using pH or catalysts,3,4 researchers have shown that spatial structuring at the microscale is possible by using light,5 catalytic surfaces,6 electrochemistry,7,8 reaction–diffusion9 and very recently, charged polymer brushes.10 However, such approaches need the aid of masks to create patterns, secondary supporting networks to act as reservoirs for gelator precursors or micro-contact printing to template micropatterns. Hence, new techniques allowing localized control over hydrogel formation and structuring can pave the way for novel soft material fabrication and patterning. Electrochemical approaches for hydrogel study and formation established so far, although limited to only a few reports, have shown promise due to their ease of use, reversibility and multiplexing ability.7,11,12 In this work, we take this approach to the next level by demonstrating localized control over hydrogel formation. This is achieved with the help of a polarized liquid–liquid interface to deposit Pt NPs on a conducting substrate of fluorine doped tin oxide (FTO) electrodes. The polarized liquid–liquid interface also known as the interface between two immiscible electrolyte solutions (ITIES) is soft, renewable, free from defects and allows the electrochemical study of interfacial ions or electron transfer processes. The liaison between ITIES and functional materials is a relatively new and unexplored topic.13 The ion or electron transfer reactions or the self-assembly process can lead to interfacial region modification with a number of functional materials (e.g. molecular sieves, liquid mirrors, and metal catalysts).14–16 When the ITIES is in contact with the conducting support a so-called three phase junction is formed. At the junction where the three phases meet, the interfacial ion transfer coupled to a redox reaction can result in very precise electrochemical deposition (e.g. the formation of Au NPs or silica).17–19 In this work, we have employed this strategy to obtain patterned (rings, stripes and spots) hydrogels by controlled formation of Pt NPs that will act as catalysts for the electrochemical splitting of water to produce a proton gradient. Controlled and localized Pt NP deposition at the micro-meter scale is facilitated by the ITIES (micro-capillary supported liquid–liquid interface) or by a three phase junction system. The proton gradient thus produced causes the self-assembly of the hydrogelator dibenzoyl-L-cystine (DBC). For simplicity, we have chosen an off-the shelf gelator as a model molecule. We believe that this technology platform provides a solid foundation for future electrochemically assisted deposition and writing of supramolecular hydrogels.
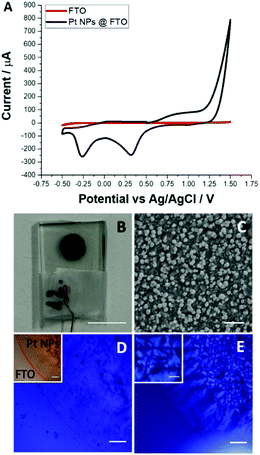
First, we focus on the demonstrating that an electrocatalytic effect of Pt NPs results in hydrogel formation. The FTO electrode placed in an electrochemical cell (experimental details are available in Section S2.1 of the ESI†) was contacted via a circular opening to a 1 mM aqueous solution of K2PtCl6 and the Pt NPs were deposited via chronoamperometry for a period of 10 min. The potential applied (E = −1.5 V vs. Ag/AgCl) was sufficient to reduce PtCl62− to metallic Pt according to reactions (1) and (2):
| PtIVCl62− + 2e− → PtIICl42− + 2Cl− | (1) |
| PtIICl42− + 2e− → Pt0 + 4Cl− | (2) |
| 2H2O − 4e− → 4H+ + O2 | (3) |
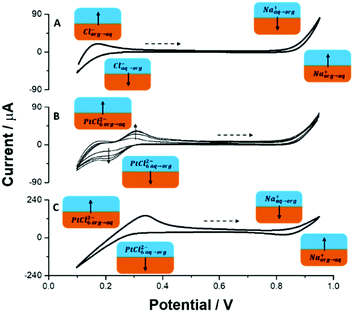
Fig. 2C shows the recordings when the organic phase contained only 5 mM PtCl6(BTPPA)2 (the concentration used in further experiments) and shows the potential window, which is now limited at the negative potential side by the PtCl6,aq↔org2−. The concept of a three phase junction modification was mainly studied by the group of Opallo, where Au NPs or silica materials were electrochemically generated in a defined locus.17–19 To the best of our knowledge, this is the first time that the three phase junction is decorated with Pt NPs. The modification follows a few mutually related ion and electron transfer reactions which are depicted together in the electrical scheme in Fig. 3. First a reductive potential is applied to the FTO electrode and the PtCl62− present in the organic phase undergoes reduction to metallic Pt (according to reactions (1) and (2)) at the three phase junction. For an applied potential (E = −1.5 V) its reduction in the organic phase is unlikely due to the very high resistance of the circuit (the high resistivity of the organic phase and the position of the reference electrode), and is therefore excluded. The Cl− formed within the junction or on the organic side of the ITIES will partition to the aqueous phase due to its high intrinsic hydrophilicity:
ClITIES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) or or![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) org− → Claq− org− → Claq−
| (4) |
| BF4,aq− → BF4,org− | (5) |
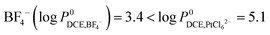 – as mentioned in Section 3 of the ESI†23 we found that for experimental concentrations of [BF4,aq−] = 100 mM and [PtCl62−] = 5 mM the latter was partitioned from the organic to the aqueous phase (a clear colour change of the aqueous phase was observed over time):
– as mentioned in Section 3 of the ESI†23 we found that for experimental concentrations of [BF4,aq−] = 100 mM and [PtCl62−] = 5 mM the latter was partitioned from the organic to the aqueous phase (a clear colour change of the aqueous phase was observed over time):| PtCl6,org2− → PtCl6,aq2− | (6) |
 | ||
| Fig. 3 A schematic representation of the three phase junction system. Organic phase is 1,2-dichloroethane. For details refer to the text. | ||
In the first two designs, the formation of Pt NPs occurs at the three-phase junction while in the third design, this junction is absent and the Pt NPs are produced by the transfer of PtCl62− from the organic to aqueous phase followed by its reduction at the FTO substrate. The micro-patterns reach in Pt NPs result from the designs in the form of stripes (ca. 175 μm), rings (ca. 1.5 mm diameter, 225 μm thick rim) and spots (ca. 60 μm diameter) respectively (Fig. 4 panel B). It was also observed that the width of the pattern was dependent on the deposition time (data not shown). The presence of the Pt NPs was confirmed in all three cases via SEM (Fig. S8, ESI†) in combination with EDS elemental mapping (inset Fig. 4 row B).
The formation of hydrogels from these patterns was carried out using linear sweep voltammetry (see Section S2.6 of ESI†). It can be seen (Fig. 4 image 1D, 2D and 3D) that there is birefringence under cross-polarization indicating the formation of oriented fibres. The time evolution of the hydrogel pattern together with a linear sweep voltammogram is shown in Fig. S10 (ESI†). From the nature of the polarization pattern, it can be concluded that the DBC fibres are oriented radially outward based on the diffusion of the protons (towards a bulk phase) produced at the interface of the Pt NP catalyst and the aqueous gelator environment. The produced hydrogels retain their shape of the base micro-pattern while being broader. Especially interesting is the hydrogel deposition achieved over a micro-spot as shown in Fig. 4 panel 3. As the region filled with Pt NPs has a micrometre size, it resembles a single microdisc electrode, in which the mass transport (to and from the electrode) will be governed by a hemispherical diffusion. The very prominent and clear Maltese cross patterns indicate that the formed hydrogel has radial orientation and probably a dome-like shape. This observation is in line with similar hemispherical shapes of silica,24–26 metallic27 or polymeric28 deposits obtained over an array of nano- and micro-electrodes.
In summary, electrochemically controlled Pt NP deposition proposed in this work was used to produce sophisticated hydrogel patterns by combining them with supramolecular hydrogelators. A polarized liquid–liquid interface was used to control the localization of Pt NPs, thereby creating new opportunities to design complicated and unusual gel patterns in a very straightforward manner. The electrocatalytic effect of Pt NPs on water oxidation allowed a local pH change which triggered the growth of a structured hydrogel directly from the support. Electrochemical patterning of hydrogels using the technique developed in this work can be used as a platform to create functional soft matter down to a micro- or nano-metre scale. As such, it opens up new directions for gel processing with potential applications in cell culturing, drug delivery, tissue engineering, sensing, diagnostics etc. Such an approach may be used to produce nano-patterned electro responsive surfaces capable of templating hydrogels and other functional soft materials.
The authors thank the NanoNextNL (a Dutch consortium of 130 companies, universities, knowledge institutes and university medical centres) Program 7A for funding. The authors thank Bart Boshuizen for his help with XPS characterization.
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611–614 CrossRef CAS.
- A. Friggeri, B. L. Feringa and J. van Esch, J. Controlled Release, 2004, 97, 241–248 CrossRef CAS.
- J. Boekhoven, J. M. Poolman, C. Maity, F. Li, L. van der Mee, C. B. Minkenberg, E. Mendes, J. H. van Esch and R. Eelkema, Nat. Chem., 2013, 5, 433–437 CrossRef CAS.
- F. Trausel, F. Versluis, C. Maity, J. M. Poolman, M. Lovrak, J. H. Van Esch and R. Eelkema, Acc. Chem. Res., 2016, 49, 1440–1447 CrossRef CAS.
- C. Maity, W. E. Hendriksen, J. H. van Esch and R. Eelkema, Angew. Chem., Int. Ed., 2015, 127, 1012–1015 CrossRef.
- A. G. L. Olive, N. H. Abdullah, I. Ziemecka, E. Mendes, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2014, 53, 4132–4136 CrossRef CAS.
- Y. Liu, E. Kim, R. V. Ulijn, W. E. Bentley and G. F. Payne, Adv. Funct. Mater., 2011, 21, 1575–1580 CrossRef CAS.
- J. Raeburn, B. Alston, J. Kroeger, T. O. McDonald, J. R. Howse, P. J. Cameron and D. J. Adams, Mater. Horiz., 2014, 1, 241–246 RSC.
- M. Lovrak, W. E. J. Hendriksen, C. Maity, S. Mytnyk, V. van Steijn, R. Eelkema and J. H. van Esch, Nat. Commun., 2017, 8, 15317 CrossRef CAS.
- Y. Wang, S. Oldenhof, F. Versluis, M. Shah, K. Zhang, V. van Steijn, X. Guo, R. Eelkema and J. H. van Esch, Small, 2019, 1804154 CrossRef.
- E. R. Cross and D. J. Adams, Soft Matter, 2019, 15, 1522–1528 RSC.
- M. Zelzer and R. V. Ulijn, Chem. Soc. Rev., 2010, 39, 3351–3357 RSC.
- L. Poltorak, A. Gamero-Quijano, G. Herzog and A. Walcarius, Appl. Mater. Today, 2017, 9, 533–550 CrossRef.
- L. Poltorak, K. Morakchi, G. Herzog and A. Walcarius, Electrochim. Acta, 2015, 179, 9–15 CrossRef CAS.
- E. Smirnov, P. Peljo, M. D. Scanlon, F. Gumy and H. H. Girault, Nanoscale, 2016, 8, 7723–7737 RSC.
- T. J. Stockmann, L. Angel, V. Brasiliense, C. Combellas and F. Kanoufi, Angew. Chem., Int. Ed., 2017, 56, 13493–13497 CrossRef CAS.
- J. Niedziolka and M. Opallo, Electrochem. Commun., 2008, 10, 1445–1447 CrossRef CAS.
- I. Kaminska, M. Jonsson-niedziolka, A. Kaminska, M. Pisarek, R. Ho, M. Opallo and J. Niedziolka-jonsson, J. Phys. Chem. C, 2012, 116, 22476–22485 CrossRef CAS.
- I. Kaminska, J. Niedziolka-jonsson, A. Roguska and M. Opallo, Electrochem. Commun., 2010, 12, 1742–1745 CrossRef CAS.
- D. Huang, B. Zhang, J. Bai, Y. Zhang, G. Wittstock, M. Wang and Y. Shen, Electrochim. Acta, 2014, 130, 97–103 CrossRef CAS.
- I. Ziemecka, G. J. M. Koper, A. G. L. Olive and J. H. van Esch, Soft Matter, 2013, 9, 1556 RSC.
- D. W. M. Arrigan, Analyst, 2004, 129, 1157–1165 RSC.
- M. Suzuki, S. Kihara, K. Maeda, K. Ogura and M. Matsui, J. Electroanal. Chem., 1990, 292, 231–244 CrossRef CAS.
- Y. Liu, A. Holzinger, P. Knittel, L. Poltorak, A. Gamero-Quijano, W. D. A. Rickard, A. Walcarius, G. Herzog, C. Kranz and D. W. M. Arrigan, Anal. Chem., 2016, 88, 6689–6695 CrossRef CAS.
- L. Poltorak, G. Herzog and A. Walcarius, Langmuir, 2014, 30, 11453–11463 CrossRef CAS.
- A. Holzinger, G. Neusser, B. J. J. Austen, A. Gamero-Quijano, G. Herzog, D. W. M. Arrigan, A. Ziegler, P. Walther and C. Kranz, Faraday Discuss., 2018, 210, 113–130 RSC.
- C. Zhu, G. Meng, Q. Huang, Z. Li, Z. Huang, M. Wang and J. Yuan, J. Mater. Chem., 2012, 22, 2271–2278 RSC.
- Y. Sakurai, S. Okuda, H. Nishiguchi, N. Nagayama and M. Yokoyama, J. Mater. Chem., 2003, 13, 1862–1864 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Methods and materials, protocols for organic salt preparation, details pertaining to electrochemical set-ups, water–1,2-dichloroethane partition coefficient calculations, and additional XPS, SEM and TEM results. See DOI: 10.1039/c9cc04238e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |