Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The key to controlling the morphologies of quantum nanocrystals: spherical carborane ligands†
Abhishek
Saini
,
Arpita
Saha
,
Clara
Viñas
 and
Francesc
Teixidor
and
Francesc
Teixidor
 *
*
Institut de Ciencia de Materials de Barcelona, Campus de la UAB, Bellaterra, 08193, Spain. E-mail: teixidor@icmab.es
First published on 25th July 2019
Abstract
By minor structure modification of spherical carborane ligands, in a similar synthetic procedure, large morphological changes are produced in Quantum Nanocrystals (QNCs). The spheres are icosahedral C2B10H12 molecules with binding sites on the carbon, and the QNCs produced are Quantum Dots, Rods, Rings and Tetrapods. These QNCs demonstrated high stability and impeccable emissive properties for more than a year.
Carboranes have geometrical figures that correspond to deltahedra having atoms only in the periphery, the more stable being the icosahedron.1a,b The icosahedral boron clusters, [B12H12]2−, [CB11H12]−, 1,2-C2B10H12 or 1,7-C2B10H12 have spherical structures and are more readily available than the geometrically similar hydrocarbon dodecahedrane (C20H20), which is very difficult to synthesize.1c Contrastingly, the icosahedral boranes can be synthesized in a single step.1d Their properties make them very attractive for material's science, particularly by the monolayer mode of packing when placed on a surface, either hexagonal or square.1e Placing these spheres in closed packing generates openings which facilitate the movement of ions and molecules.2a The properties of the carboranyl spheres were further used to link them to the binding sites of Quantum Dots (QDs) to produce CdSe QDs with a novel core canopy architecture.2b
The relevance of ligands in the synthesis of Quantum Nanocrystals (QNCs) is well established.3 The ligands, which are commonly hydrophobic organic molecules, prevent the formation of a bulk semiconductor material by coordination with the unsaturated metal atoms on the surface of QDs.4 The size of the quantum dot is determined by the crystal growth time.4 Quantum Rods (QRods)5 and Quantum Rings (QRs)6 have also been synthesized using colloidal procedures and using ligands as capping agents for shape modification, though to date these procedures are highly complex and can’t match the ease with which these QRs and QRods can be synthesized by physical methods. The electronic and optoelectronic properties of nanocrystals depend strongly on the shape of the nanostructure.7 In this regard, QRs are unique nanostructures with unique properties because of their circular geometry.8 To study these fascinating nano-structures, efficient methods to produce QRs are necessary. But for one reported case of colloidal synthesis that requires etching,6 QRs are currently made by physical methods using electron beam lithography, and ion beam milling.9 Distinct to QRs, QRods are of great interest due to their elongated lengths providing a number of advantages over the traditional QDs and their own set of unique properties.10 These make QRods highly desirable in the field of applications like in fluorescent labels and markers but they experience low throughput as QRs when prepared using physical methods.
To produce different morphologies of QNCs following a colloidal procedure, we relied on unconventional ligands with spheres having an appended coordinating site, i.e., carboranyl ligands. The reason behind this is that spheres can ideally pack in a compact way with a hexagonal or square arrangement, thus influencing the inner core by the outer sphere packing. Here, we have used a standard colloidal synthetic method,11 emphasizing on the ligand used, to synthesize QNCs with different morphologies, and high throughput. QDs, QRods, QRs, and Quantum Tetrapods (QTPs) have been produced. Seven carborane derivatives12 have been studied in this work as depicted in Fig. S1 (ESI†), and described in Table 1. The synthetic procedure used is a modified version of a previously reported one.13
| Carborane derivative acting as a capping agent | Quantum-nanocrystal morphology | Colors of emission |
|---|---|---|
| meta-Carboranethiol | QDs | Blue to yellow |
| ortho-Carboranethiol | QRods | Blue to yellow |
| meta-Carboranedithiol | QRods | Shades of yellow |
| 1-Methyl-ortho-2-carboranethiol | QRs | Blue to yellow |
| meta-Carboranephosphinic acid | QRs | Blue and green |
| meta-Carboranediphosphinic acid | QRs | Blue and green |
| meta-Carboranecarboxylic acid | QTPs | Green and yellow |
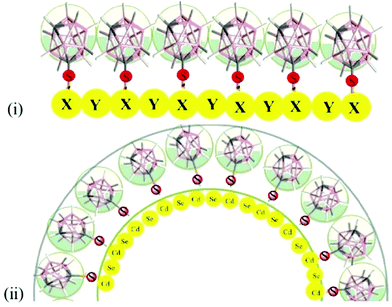
Our approach assumed that the compactness of the arranged spherical carborane units would create curvatures. The concept is schematically shown in Fig. 1. The scheme in Fig. 1(i) implies a perfect matching of the sphere's van der Waals diameter and the covalent diameter of the Cd–Se. However, if this matching is good, but not perfect, as the two diameters are 656 pm vs. 528 pm respectively, then the only way for them to fit with each other would be through the model shown in Fig. 1(ii), i.e., the two concentric circles.
To perform the synthesis, it was needed to carefully control the temperature and its ramps. If the temperature is not ramped up and increased stepwise, independently of the carborane used, perfectly spherical shaped QDs are produced. These QDs have sizes in the range of 2 to 3 nm in diameter with varying colours depending on the elapsed time of pipetting of the samples. Upon incorporating a sudden increase in temperature for the last 40–50 °C, we observe the formation of differently shaped NCs, depending upon the ligand used. It must be pointed out that meta-carboranethiol always produces spherical QDs irrespective of the sudden temperature increase. Table 1 lists the different morphologies formed with their respective capping agent.
The abrupt rise in temperature from 150 °C to 195 °C has already been reported as necessary to produce QRods with TOPO/Oleic Acid.14 We suggest that this abrupt temperature change induces the formation of nanobubbles that become the template to generate different morphologies other than the traditional QDs, given the appropriate ligands. After the synthesis procedure was completed, efforts were made to completely isolate the QNCs hence produced, from their colloidal suspension. After unsuccessful attempts with the use of PTFE microfilters, a modicum of success was achieved by using ethanol to highly dilute the colloidal suspension and then centrifuging it as seen in Fig. S2a in the ESI.†
Of particular interest amongst these QNCs is the formation of QRs and QTPs,15 the colloidal synthesis of which is extremely rare and certainly has never been achieved before with such ease in a one-step synthesis. The different QNCs formed are shown in Fig. 2, while Fig. S2b in the ESI† depicts the facile growth of the QRs after Se addition.
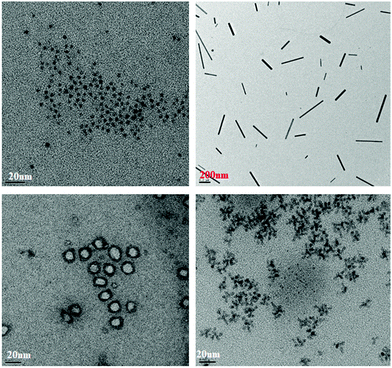 | ||
| Fig. 2 The different shapes are shown in this figure. The top left corner shows the QDs, the top right shows the QRods, the bottom left shows the QRs and the bottom right shows the QTDs. | ||
The TEM, STEM and HRTEM images of the QNCs are shown in Fig. S3 and S4, ESI.† The existence of B, Cd, Se and P or S in these QNCs was proven by spectroscopic techniques. The presence of Boron indicating carborane was detected by EELS in all samples of QRs, QDs, QRods and the QTPs (Fig. S5–S11, ESI†). The B–H band appears around 2500 cm−1 near the range of S–H, but the disappearance of the S–H band in the QDs has already been reported16 (Fig. S12–S14, ESI†). For QRs, the ratio Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se is 2
Se is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This is not unexpected as traditionally in most QDs the Cd
1. This is not unexpected as traditionally in most QDs the Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratio is skewed in favour of Cd, but not so much as to reach a well-defined 2
Se ratio is skewed in favour of Cd, but not so much as to reach a well-defined 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The EDX of all QNCs shows a ratio of Cd
1. The EDX of all QNCs shows a ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se as 2
Se as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but for the QTPs that is of 3
1, but for the QTPs that is of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This ratio of Cd
2. This ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se remained constant over 5 months (Fig. S16–S22, ESI†). The ICP-MS analysis for the samples also showed the ratio of Cd
Se remained constant over 5 months (Fig. S16–S22, ESI†). The ICP-MS analysis for the samples also showed the ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se as 2
Se as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S1, ESI†). For QRs generated with meta-carborane phosphinic acid and meta-carboranediphosphinic acid, the EDX ratio of elements Cd
1 (Table S1, ESI†). For QRs generated with meta-carborane phosphinic acid and meta-carboranediphosphinic acid, the EDX ratio of elements Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se
Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P is 2
P is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
The importance of ligands in controlling morphologies of NCs in colloidal synthesis has been reported17 but the mechanism of this is still not clear. Viewed with a certain perspective, one could envisage similarities between the sphere-to-rod transitions of non-ionic surfactant micelles in aqueous solution18 and the QDs to QRods possible transition that is considered in this work. However, they are not related. In the first one, the transition is due to an increasing concentration of the same surfactant going through two CMC; in the case reported here all syntheses are carried out at the same concentration of Cd and Se precursors and the distinct carboranes. Furthermore, the solvent and temperature and temperature ramps are alike in the different processes. The only differences are the carboranes with their distinct ligating sites and non-binding substituents. Thus, it is our hypothesis that is the interplay of the hydrophobic interactions between carborane units, the electronically minor but sterically relevant carborane substituents, the sphere packings and the consequences of the mismatch described earlier that lead to the formation of these geometric features. We do not discard, however, the assistance of the nanobubble template effect which would be originated by the sudden temperature rise. This is conjectural but may be an explanation to account for the necessity of abrupt temperature increase to generate different QNCs, as opposed to the traditional QDs if the abrupt temperature rise does not take place. Electron diffraction studies confirmed the synthesis of CdSe materials with a hexagonal phase (Fig. S23, ESI†), in all the QNCs. The QRods and QTPs had a more crystalline diffraction pattern while the QRs and the QDs had a more amorphous diffraction pattern. Fig. S26 (ESI†) shows the mean size of the QDs, QRods and QRs formed. They are all well within the quantum regime of sizes. To substantiate the relevance of the spheres in the formation of QNCs we compared the carboranethiol with adamantanethiol by using the same conditions of synthesis leading to QNCs. The result was the formation of QDs with a weak fluorescence. No other morphology was found proving the necessity of the carborane ligands in producing these novel structures (Fig. S25a and b, ESI†).
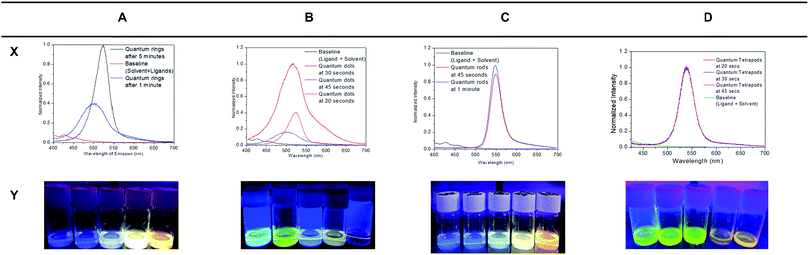
The fluorescence intensities of the QNCs produced using carborane derivatives are far superior to the QDs commonly synthesized in an organic medium capped with TOPO and TOP. The carborane capped QNCs are fluorescent and maintain their lifetime of fluorescence for up to a year as tested. The intensity of QRs, QRods and QDs are measured using filters, which only allow for 1% of the total intensity of emission to be recorded, as the intensity of these QNCs was too high to be recorded by the arbitrary units of the fluorometer. The fluorescence colours shown by these QNCs vary greatly, depending upon the different ligands used and also upon the time of pipetting out of the sample from the reaction mixture. Based upon the time elapsed of the reaction after Se addition, the QNCs capped by the same ligand can show different colours (Table 1).
The QRs with 1-methyl-ortho-2-carboranethiol emit colours from blue to yellow suggesting a facile growth process with time (see Fig. S1b, ESI†), while the QRs with meta-carborane phosphinic acid and meta-carboranediphosphinic acid show mainly green and bluish colour that suggests a less favourable growth process. The QDs made with meta-carboranethiol emit different shades ranging from green to yellow. The QRods capped with the ortho-carboranethiol emit colours from blue to yellow while the QRods capped with meta-carboranedithiol emit shades of yellow. In terms of luminescence, the most curious property is shown by the QTPs capped with meta-carboranecarboxylic acid. While the emission of the rest of the QNCs remains constant after being pipetted out, irrespective of their storage time, the QTPs show variance in colours even after they have been pipetted out of the reaction mixture. Their emission keeps on changing over the course of time in storage. They emit green colour on the day of the reaction, which changes to yellow after 2 months and to orange after 5 months. This change in colour would normally suggest that the QTPs keep growing in size over time or aggregating, but TEM images show no increase in size even after 5 months of storage. The EDX analysis also showed that the chemical composition of these QTPs stays constant over 5 months (Fig. S22, ESI†). The change in colour seems to be independent of the size and chemical composition. Our tentative explanation is that different phases are generated as a result of the ageing of the samples which leads to this colour change. In support of this, we found that the recently formed QTPs have the hexagonal wurtzite structure while the older QTPs show a cubic zinc blende phase (Fig. S23b and S24a, ESI†). The fluorescence intensities are further represented in Fig. 3, with baseline intensity already accounted for. The absorption spectra are recorded and they lie within the 300 to 400 nm range with maximum absorption around 360 nm (Fig. S27–S29, ESI†).
In summary, this paper demonstrates the ease with which we can control the morphology of various QNCs, all the while following the same colloidal synthesis procedure, by incorporating spherical carborane ligands as capping agents. The QNCs hence produced are all highly stable, and show high fluorescence intensities. The use of icosahedral carboranes of formula C2B10H12 for the synthesis of QRs, QRods and QTPs is unprecedented and highlights the relevance of the spherical ligands. This synthetic procedure can facilitate a shift in focus from trying to find a viable synthesis procedure for morphologies such as QRs and QTPs, towards studying them and their properties in further detail and producing them in sufficient quantities for use in novel applications.
This work has been supported by the Spanish Ministerio de Economia y Competitividad (CTQ2016-75150-R) and Generalitat de Catalunya (2014/SGR/149). A. Saha thanks Generalitat de Catalunya for FPI predoctoral grant (2016FI_B 00214). A. Saini and A. Saha are enrolled in the PhD in Chemistry program of UAB.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. Poater, M. Solà, C. Viñas and F. Teixidor, Angew. Chem., Int. Ed., 2014, 53, 12191–12195 CrossRef CAS; (b) J. Poater, M. Solà, C. Viñas and F. Teixidor, Chem. – Eur. J., 2016, 22, 7437–7443 CrossRef CAS PubMed; (c) L. A. Paquette, R. J. Ternansky, D. W. Balogh and G. Kentgen, J. Am. Chem. Soc., 1983, 105, 4446–4450 CrossRef; (d) R. Caputo, S. Garroni, D. Olid, F. Teixidor, S. Suriñach and M. D. Baro, Phys. Chem. Chem. Phys., 2010, 12, 15093 RSC; (e) R. N. Grimes, Carboranes, Elsevier, Oxford, 3rd edn, 2016 Search PubMed.
- (a) A. M. Cioran, A. D. Musteti, F. Teixidor, Ž. Krpetić, I. A. Prior, Q. He, C. J. Kiely, M. Brust and C. Viñas, J. Am. Chem. Soc., 2012, 134, 212–221 CrossRef CAS PubMed; (b) A. Saha, E. Oleshkevich, C. Vinas and F. Teixidor, Adv. Mater., 2017, 29, 1704238 CrossRef PubMed.
- (a) A. P. Alivisatos, W. Gu and C. Larabell, Annu. Rev. Biomed. Eng., 2005, 7, 55–76 CrossRef CAS PubMed; (b) J. Weng and J. Ren, Curr. Med. Chem., 2006, 13, 897–909 CrossRef CAS.
- S. N. Raja, A. C. K. Olson, K. Thorkelsson, A. J. Luong, L. Hsueh, G. Chang, B. Gludovatz, L. Lin, T. Xu, R. O. Ritchie and A. P. Alivisatos, Nano Lett., 2013, 13, 3915–3922 CrossRef CAS PubMed.
- (a) A. Wolcott, R. C. Fitzmorris, O. Muzaffery and J. Z. Zhang, Chem. Mater., 2010, 22, 2814–2821 CrossRef CAS PubMed; (b) A. E. Saunders, F. Shieh and B. A. Korgel, J. Phys. Chem. B, 2005, 109, 8538–8542 CrossRef PubMed; (c) K. Manthiram, B. J. Beberwyck, D. V. Talapin and A. P. Alivisatos, J. Visualized Exp., 2013, 82, 50731 Search PubMed.
- I. Fedin and D. V. Talapin, J. Am. Chem. Soc., 2016, 138, 9771–9774 CrossRef CAS PubMed.
- K. D. Gilroy, A. Ruditskiy, H. C. Peng, D. Qin and Y. Xia, Chem. Rev., 2016, 116, 10414–10472 CrossRef CAS PubMed.
- (a) C. Tong, S. Yoon and L. Wang, Nanoscale Res. Lett., 2012, 7, 520 CrossRef PubMed; (b) V. E. Demidov, S. Urazhdin, G. de Loubens, O. Klein, V. Cros, A. Anane and S. O. Demokritov, Phys. Rep., 2017, 673, 1–31 CrossRef CAS; (c) A. Fuhrer, S. Lüscher, T. Ihn, T. Heinzel, K. Ensslin, W. Wegscheider and M. Bichler, Nature, 2001, 413, 822–825 CrossRef CAS PubMed; (d) W. C. Tan and J. C. Inkson, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 5626–5635 CrossRef CAS; (e) V. I. Klimov, S. A. Ivanov, J. Nanda, M. Achermann, I. Bezel, J. A. McGuire and A. Pirystinski, Nature, 2007, 447, 441–446 CrossRef CAS PubMed; (f) G. O. De Sousa, D. R. Da Costa, A. Chaves, G. A. Farias and F. M. Peeters, Phys. Rev. B, 2017, 95, 20514 CrossRef.
- (a) A. Lorke, R. J. Luyken, M. Fricke, J. P. Kotthaus, G. Medeiros-Ribeiro, J. M. Garcia and P. M. Petroff, Microelectron. Eng., 1999, 47, 95–99 CrossRef CAS; (b) A. Lorke, R. J. Luyken, A. O. Govorov, J. P. Kotthaus, J. M. Garcia and P. M. Petroff, Phys. Rev. Lett., 2000, 84, 2223–2226 CrossRef CAS PubMed; (c) D. Haft, F. Bickel, A. Lorke, K. Karrai, J. M. Garcia, W. Schoenfeld and P. M. Petroff, Nature, 2000, 405, 8–11 CrossRef PubMed; (d) C. Somaschini, S. Bietti, N. Koguchi and S. Sanguinetti, Nanotechnology, 2011, 22, 185602 CrossRef CAS PubMed; (e) C. Somaschini, S. Bietti, S. Sanguinetti, N. Koguchi and A. Fedorov, Nanotechnology, 2010, 21, 125601 CrossRef CAS PubMed.
- (a) L. Li, J. Hu, W. Yang and A. P. Alivisatos, Nano Lett., 2001, 1, 349–351 CrossRef CAS; (b) J. Hu, L. S. Li, W. Yang, L. Manna, L. W. Wang and A. P. Alivisatos, Science, 2001, 292, 2060–2063 CrossRef CAS PubMed; (c) A. Shabaev and A. L. Efros, Nano Lett., 2004, 4, 1821–1825 CrossRef CAS; (d) H. Htoon, J. A. Hollingworth, A. V. Malko, R. Dickerson and V. I. Klimov, Appl. Phys. Lett., 2003, 82, 4776–4778 CrossRef CAS; (e) E. Rothenberg, M. Kazes, E. Shaviv and U. Banin, Nano Lett., 2005, 5, 1581–1586 CrossRef CAS PubMed.
- (a) B. O. Dabbousi, J. Rodriguez-Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463–9475 CrossRef CAS; (b) I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS PubMed.
- (a) C. Viñas, R. Benakki, F. Teixidor and J. Casabó, Inorg. Chem., 1995, 34, 3844–3845 CrossRef; (b) E. Oleshkevich, F. Teixidor, D. Choquesillo-Lazarte, R. Sillanpää and C. Viñas, Chem. – Eur. J., 2016, 22, 3665–3670 CrossRef CAS PubMed; (c) M. Fontanet, A. R. Popescu, X. Fontrodona, M. Rodriguez, I. Romero, F. Teixidor, C. Viñas, N. A. Alcalde and E. Ruiz, Chem. – Eur. J., 2011, 17, 13217–13229 CrossRef CAS PubMed.
- C. A. Ubani and S. M. Yusof, J. Mod. Educ. Rev., 2011, 1, 63–73 Search PubMed.
- W. J. Baumgardner, Z. Quan, J. Fang and T. Hanrath, Nanoscale, 2012, 4, 3625 RSC.
- (a) T. Mokari, E. Rothenburg, I. Popov, R. Costi and U. Banin, Science, 2004, 304, 1787–1790 CrossRef CAS PubMed; (b) L. Manna, D. J. Miliron, A. Meisel, E. C. Scher and A. P. Alivisatos, Nat. Mater., 2003, 2, 382–385 CrossRef CAS PubMed.
- A. E. Vikraman, A. R. Jose, M. Jacob and K. G. Kumar, Anal. Methods, 2015, 7, 6791–6798 RSC.
- (a) A. Y. Koposov, CrystEngComm, 2017, 19, 3098–3102 RSC; (b) W. W. Yu, Y. A. Wang and X. Peng, Chem. Mater., 2003, 15, 4300–4308 CrossRef CAS.
- M. Velinova, D. Sengupta, A. V. Tadjer and S. J. Marrink, Langmuir, 2011, 27, 14071–14077 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The structure of different ligands, schematic of synthesis and TEM, STEM and HRTEM images, UV-vis absorbance, IR, EELS, EDX spectra, electron diffraction patterns, ICP-MS analysis and detailed experimental procedure. See DOI: 10.1039/c9cc03941d |
| This journal is © The Royal Society of Chemistry 2019 |