Open Access Article
Open Access ArticleActivity-based protein profiling reveals that secondary-carbon-centered radicals of synthetic 1,2,4-trioxolanes are predominately responsible for modification of protein targets in malaria parasites†
Chunyan
Wei‡
a,
Cheng-Xiao
Zhao‡
bc,
Sheng
Liu‡
b,
Jin-Hui
Zhao‡
b,
Zi
Ye
b,
Heng
Wang
*ab,
Shi-Shan
Yu
*b and
Chong-Jing
Zhang
 *b
*b
aDepartment of Microbiology and Parasitology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & School of Basic Medicine, Peking Union Medical College, 5# Dong Dan San Tiao, Beijing, 100005, China. E-mail: wanghpumc@163.com
bState Key Laboratory of Bioactive Substances and Functions of Natural Medicines and Beijing Key Laboratory of Active Substances Discovery and Drugability Evaluation, Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, 100050, China. E-mail: yushishan@imm.ac.cn; zhangchongjing@imm.ac.cn
cSchool of Pharmaceutical Science, Shanxi Medical University, Taiyuan, Shanxi 030001, China
First published on 23rd July 2019
Abstract
Endoperoxide-containing antimalarials, such as artemisinin and the synthetic trioxolane OZ439, are prodrugs activated by heme to generate primary and secondary carbon-centered radicals. We employed activity-based protein profiling (ABPP) to show that the secondary-carbon-centered radical of 1,2,4-trioxolanes is primarily responsible for protein labeling in malaria parasites.
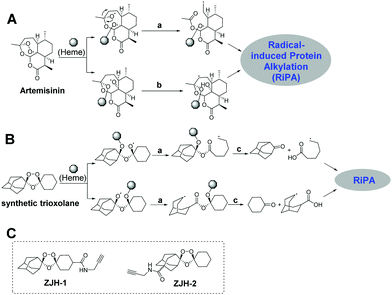
Malaria caused by the protozoal parasite Plasmodium falciparum and related species is a mosquito-borne and life-threating infectious disease, which leads to nearly a half million deaths globally each year.1 The first small molecule drug used to treat malaria was quinine, extracted from the cinchona tree.2 Due to limited access to this natural source during the Second World War, chloroquine, a synthetically-derived quinoline was identified as an anti-malaria drug.3 Chloroquine and its derivatives were extensively used from the 1930s until the 1950s.4 Unfortunately, widespread resistance to chloroquine eventually developed, prompting a search for next generation anti-malaria drugs. Chinese scientists led by Youyou Tu discovered the fascinating natural product artemisinin (Qinghaosu) and showed that it possesses unparalleled efficacy for treatment of malaria including complicated malaria caused by chloroquine-resistant plasmodial strains.5 Artemisinin established the pivotal role of the endoperoxide as a unique and effective antimalarial pharmacophore.6 The disadvantages of artemisinin are its rapid clearance in vivo, limited supply and impractical de novo synthesis. These challenges stimulated the development of synthetically-accessible and longer-acting trioxolanes, such as OZ277 and OZ439.7 Mechanism of action (MOA) studies revealed that the unique endoperoxide group in artemisinin and synthetic trioxolanes was first activated by heme to generate carbon-centered radicals, which subsequently alkylate multiple proteins in malaria parasites.8 During the activation of the endoperoxide by heme, two different radicals are generated, namely, primary and secondary carbon-centered radicals, each with their own unique properties (Fig. 1A and B).9 The primary carbon-centered radical is sterically smaller while the secondary-carbon-centered radical is more stable.10 However, the relative contributions of each radical for antimalarial activity and specific protein targets have not been elucidated. Herein we determine the protein targets of each radical and further refine our mechanistic understanding of endoperoxide-containing anti-malaria molecules.
To answer the above-mentioned mechanistic questions, we used activity-based protein profiling (ABPP) to capture the alkylated proteins of each radical. This was accomplished by designing two chemical probes containing an alkyne group. Alkynes are typically used in ABPP because they are sterically small and can be selectively conjugated to a fluorophore or an affinity tag employing bioorthogonal click-chemistry.11 Artemisinin is not an ideal substrate for these studies since both the radicals remain in the same scaffold (Fig. 1A); thus it is impossible to discern the specific proteins labeled by each radical regardless of where the alkyne group is introduced. On the other hand, the synthetic trioxolane provides an ideal scaffold for our proposed investigation. After heme-mediated activation followed by rearrangement and hydrolysis, synthetic trioxolane is transformed into either an adamantane ketone and a primary-carbon-centered radical, or a cyclohexane ketone and a secondary-carbon-centered radical (Fig. 1B). The protein targets of the two radicals can be easily traced by selecting appropriate attachment sites for the alkyne group. Following this design principle, incorporation of the alkyne into the cyclohexane part (ZJH-1, Fig. 1C) will capture the protein targets of the primary-carbon-centered radical while attachment of the alkyne group into the adamantane part (ZJH-2, Fig. 1C) will capture the protein targets of the secondary-carbon-centered radical.
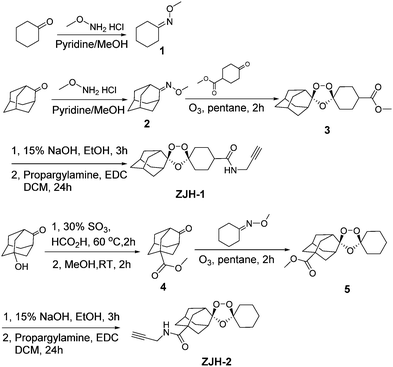
Following this rationale, we synthesized probes ZJH-1 and ZJH-2 (Scheme 1). Oxime 1 and 2 were synthesized via the reaction between the corresponding ketone and methoxyamine. The Griesebaum co-ozonolysis of 4-(methoxycarbonyl)cyclohexanone with oxime 2 afforded trioxolane 3. Saponification of 3 followed by EDC-mediated coupling with propargyl amine provided the probe ZJH-1. Probe ZJH-2 was synthesized in a similar manner as described.8c The chemical structures of probes ZJH-1 and ZJH-2 were confirmed by NMR spectroscopy and high-resolution mass spectrometry (ESI,† Fig. S1–S14).
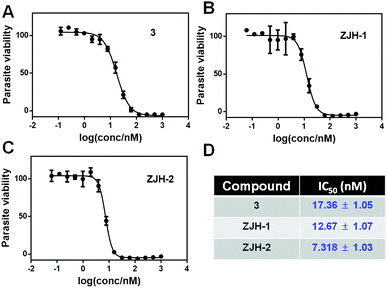
We measured the antimalarial activity of probes ZJH-1 and ZJH-2 against P. falciparum 3D7 in vitro. Synchronization of parasite cultures with two rounds of 5% (w/v) D-sorbitol was performed as previously described.12 Compound 3 was included as a positive control. The parasite sensitivity of compound 3, ZJH-1 and ZJH-2 was measured using SYBR Green I as an indicator.13 The excitation and emission wavelengths of SYBR Green I were set as 485 and 528 nm, respectively. All three compounds could effectively inhibit the parasite growth and the calculated IC50 values were 12.67 nM, 7.32 nM and 17.36 nM for ZJH-1, ZJH-2 and compound 3, respectively (Fig. 2). These results indicate that incorporation of an alkyne in probes ZJH-1 and ZJH-2 did not perturb the antimalarial activity confirming that these probes phenocopy the parent trioxolane scaffold and are thus suitable for ABPP.
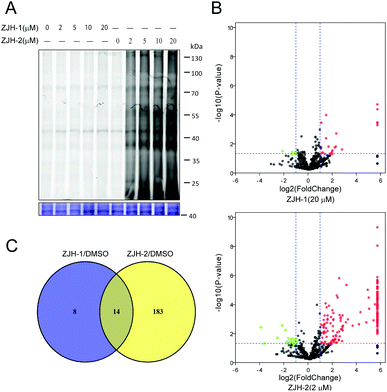
To assess the ability of probes ZJH-1 and ZJH-2 to label proteins in malaria parasites, different concentrations of probes ZJH-1 and ZJH-2 ranging from 2 to 20 μM were incubated with the mixed stage of P. falciparum parasite cultures for 4 hours at 37 °C. Red blood cells were removed by lysis with 0.1% saponin and parasites were pelleted down by centrifugation. The labeled proteome in the parasites was obtained by sonication and the probe-modified proteins were labeled with a fluorescent dye (rhodamine-azide conjugate) using standard copper-catalyzed click chemistry conditions. The treated proteome was resolved by denaturing gel electrophoresis and visualized by in-gel fluorescence scanning (Fig. 3A and ESI,† Fig. S15). We observed that ZJH-2 efficiently labeled proteins at the lowest probe concentration examined (2 μM), whereas ZJH-1 displayed virtually no labeling at this concentration and could only label a few proteins at the highest concentration evaluated (20 μM). This result indicates that the secondary-carbon-centered radical predominates protein labeling of synthetic 1,2,4-trioxolanes in malaria parasites. The poor protein labeling of the primary-carbon-centered radical could be ascribed to two causes. First, because of the steric hindrance, the coordination of heme to the endoperoxide likely occurs at the least hindered oxygen atom resulting in generation of the secondary-carbon-centered one. Second, the primary-carbon-centered radical is less stable than the secondary carbon-centered one.
To further support the result of gel-based imaging, we next employed gel-free quantitative proteomics to pull down and identify the corresponding protein targets of ZJH-1 and ZJH-2 in parasites. Briefly, the alkyne labeled parasite proteome was reacted with biotin-azide, followed by enrichment with streptavidin beads. Enriched beads were subjected to on-bead digestion with trypsin to release peptide fragments of bound proteins. Collected peptides were separated and detected by LC-MS/MS and the results were processed with the label-free proteomics method to identify the protein targets of ZJH-1 and ZJH-2. During data processing, we used a strict cut-off fold change of 2 and a p-value of 0.05 as the qualification criterion. Accordingly the probe ZJH-1 targeted 22 proteins at the concentration of 20 μM, while ZJH-2 targeted 197 proteins at the concentration of 2 μM (Fig. 3B, ESI,† sheet 6 and sheet 7 in proteomics data.xlsx). There were 14 proteins labeled by both ZJH-1 and ZJH-2 (Fig. 3C ànd ESI,† Table S1). These results confirm that the secondary-carbon-centered radical is much more effective in protein-labeling than the primary-carbon-centered one. The recent clinical study of OZ439 showed that the drug's peak plasma concentration is around 3 μM.14 Taken together, our finding suggests that the secondary-carbon-centered radical from trioxolane accounts for nearly all the alkylated proteins in vivo.
To further support the result of the chemical proteomics study, we carried out an in vitro experiment to check the products of radical-induced alkylation. Briefly, probes ZJH-1 and ZJH-2 were respectively incubated with hemin and glutathione (GSH) in the presence of sodium ascorbate. We checked the reaction by LC-MS. The expected reaction products between the probes and GSH are shown in Fig. 4A and ESI,† Fig. S17A. In the LC-MS profiles, we found that the main products generated by secondary-carbon-centered radicals (ZJH-1-P-S and ZJH-2-P-S) have a single ion peak in the mass spectra with the expected m/z ratio (Fig. 4B, ESI,† Fig. S16C, D and S18). However, it is difficult to find the ion peak for the product generated by the primary-carbon-centered radicals (ZJH-1-P-P and ZJH-2-P-P) (ESI,† Fig. S16D and S17E). This result also indicated that the secondary-carbon-centered radicals are produced much more than the primary-carbon-centered ones after the trioxolane group was activated by heme.
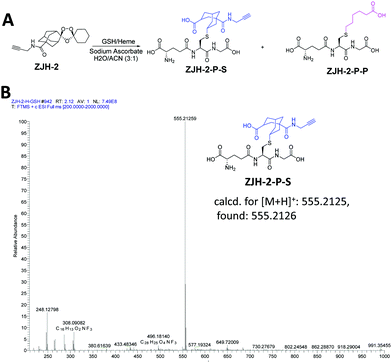 | ||
| Fig. 4 Reaction between ZJH-2 and GSH in the presence of heme. (A) Reaction scheme of ZJH-2 and GSH in the presence of heme and sodium ascorbate; (B) mass spectrum of the main product of ZJH-2-P-S. | ||
In conclusion, we designed two molecular probes to investigate the protein labeling capacity of primary and secondary carbon-centered radicals generated by synthetic trioxolanes. Both gel-based and gel-free proteomics methods demonstrated that the secondary-carbon-centered radical was responsible for nearly all the protein labeling in situ. These results provide a more nuanced insight into the unique mode of action of the endoperoxide-containing antimalarials. This study may also aid the further development of potent anti-malaria drugs.
We greatly thank Prof. Courtney Aldrich of the University of Minnesota for careful editing of our manuscript. We also thank the start-up funding from the State Key Laboratory of Bioactive Substances and Functions of Natural Medicines, Institute of Materia Medica, Peking Union Medical College, and CAMS Innovation Fund for Medical Sciences (CIFMS) (2017-I2M-4-005, 2016-I2M-1-019).
Conflicts of interest
The authors declare that there is no conflict of interest in the publication of this article.Notes and references
- https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/ .
- T. S. Kaufman and E. A. Rúveda, Angew. Chem., Int. Ed., 2005, 44, 854–885 CrossRef CAS PubMed.
- K. Krafts, E. Hempelmann and A. Skórska-Stania, Parasitol. Res., 2012, 111, 1–6 CrossRef PubMed.
- A. F. G. Slater, Pharmacol. Ther., 1993, 57, 203–235 CrossRef CAS PubMed.
- Y. Tu, Angew. Chem., Int. Ed., 2016, 55, 10210–10226 CrossRef CAS PubMed.
- (a) Z. Guo, Acta Pharm. Sin. B, 2016, 6, 115–124 CrossRef PubMed; (b) Y. Wu, Acc. Chem. Res., 2002, 35, 255–259 CrossRef CAS PubMed.
- (a) J. L. Vennerstrom, S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. Dong, A. Dorn, D. Hunziker, H. Matile, K. Mclntosh, M. Padmanilayam, J. S. Tomas, C. Scheurer, B. Scorneaux, Y. Tang, H. Urwyler, S. Wittlin and W. N. Charman, Nature, 2004, 430, 900–904 CrossRef CAS PubMed; (b) S. A. Charman, S. Arbe-Barnes, I. C. Bathurst, R. Brun, M. Campbell, W. N. Charman, F. C. K. Chiu, J. Chollet, C. Craft, D. J. Creek, Y. Dong, H. Matile, M. Maurer, J. Morizzi, T. Nguyen, P. Papastogiannidis, C. Scheurer, D. M. Shackleford, K. Sriraghavan, L. Stingelin, Y. Tang, H. Urwyler, X. Wang, K. L. White, S. Wittlin, L. Zhou and J. L. Vennerstrom, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4400–4405 CrossRef CAS PubMed.
- (a) J. Wang, C.-J. Zhang, W. N. Chia, C. C. Y. Loh, Z. Li, Y. M. Lee, Y. He, L.-X. Yuan, T. K. Lim, M. Liu, C. X. Liew, Y. Q. Lee, J. Zhang, N. Lu, C. T. Lim, Z.-C. Hua, H.-M. Shen, K. S. W. Tan and Q. Lin, Nat. Commun., 2015, 6, 10111 CrossRef CAS PubMed; (b) H. M. Ismail, V. Barton, M. Phanchana, S. Charoensutthivarakul, M. H. L. Wong, J. Hemingway, G. A. Biagini, P. M. O’Neill and S. A. Ward, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2080–2085 CrossRef CAS PubMed; (c) H. M. Ismail, V. Barton, M. Phanchana, S. Charoensutthivarakul, G. A. Biagini, S. A. Ward and P. M. O’Neill, Angew. Chem., Int. Ed., 2016, 55, 6401–6405 CrossRef CAS PubMed; (d) C.-J. Zhang, J. Wang, J. Zhang, Y. M. Lee, G. Feng, T. K. Lim, H.-M. Shen, Q. Lin and B. Liu, Angew. Chem., Int. Ed., 2016, 55, 13770–13774 CrossRef CAS PubMed; (e) W. Li, Y. Zhou, G. Tang and Y. Xiao, Bioconjugate Chem., 2016, 27, 2828–2833 CrossRef CAS PubMed.
- Y. Tang, Y. Dong, X. Wang, K. Sriaghavan, J. K. Wood and J. L. Vennerstrom, J. Org. Chem., 2005, 70, 5103–5110 CrossRef CAS PubMed.
- J. Hioe and H. Zipse, Org. Biomol. Chem., 2010, 8, 3609–3617 RSC.
- (a) C. Drahl, B. F. Cravatt and E. J. Sorensen, Angew. Chem., Int. Ed., 2005, 44, 5788–5809 CrossRef CAS PubMed; (b) T. F. Gronauer, M. M. Mandl, M. Lakemeyer, M. W. Hackl, M. Meßner, V. S. Korotkov, J. Pachmayr and S. A. Siever, Chem. Commun., 2018, 54, 9833–9836 RSC; (c) Z. Li, P. Hao, L. Li, C. Y. J. Tan, X. Cheng, G. Y. J. Chen, S. K. Sze, H.-M. Shen and S. Q. Yao, Angew. Chem., Int. Ed., 2013, 52, 8551–8556 CrossRef CAS PubMed; (d) H. Guo, J. Xu, P. Hao, K. Ding and Z. Li, Chem. Commun., 2017, 53, 9620–9623 RSC; (e) H. Park, J. Y. Koo, Y. V. Srikanth, S. Oh, J. Lee, J. Park and S. B. Park, Chem. Commun., 2016, 52, 5828–5831 RSC; (f) N. Chen, J. Liu, Z. Qiao, Y. Liu, Y. Yang, C. Jiang, X. Wang and C. Wang, Chem. Sci., 2018, 9, 2826–2830 RSC.
- C. Lambros and J. P. Vanderberg, J. Parasitol., 1979, 65, 418–420 CrossRef CAS PubMed; S. Pan, H. Zhang, C. Wang, S. C. L. Yao and S. Q. Yao, Nat. Prod. Rep., 2016, 33, 612–620 RSC.
- (a) T. N. Bennett, M. Paguio, B. Gligorijevic, C. Seudieu, A. D. Kosar, E. Davidson and P. D. Roepe, Antimicrob. Agents Chemother., 2004, 48, 1807–1810 CrossRef CAS PubMed; (b) M. Smilkstein, N. Sriwilaijaroen, J. X. Kelly, P. Wilairat and M. Riscoe, Antimicrob. Agents Chemother., 2004, 48, 1803–1806 CrossRef CAS PubMed.
- A. P. Phyo, P. Jittamala, F. Hosten, S. Pukrittayakamee, M. Imwong, N. J. White, S. Duparc, F. Macintyre, M. Baker and J. J. Möhrle, Lancet Infect. Dis., 2016, 16, 61–69 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Intermediates and methods, and supplementary and extended figures. See DOI: 10.1039/c9cc03719e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |