Open Access Article
Open Access ArticleSwitching the enantioselectivity of nanoporous host materials by light†
Anemar Bruno
Kanj
 a,
Jochen
Bürck
a,
Jochen
Bürck
 b,
Sylvain
Grosjean
b,
Sylvain
Grosjean
 c,
Stefan
Bräse
cde and
Lars
Heinke
c,
Stefan
Bräse
cde and
Lars
Heinke
 *a
*a
aKarlsruhe Institute of Technology (KIT), Institute of Functional Interfaces (IFG), 76344 Eggenstein-Leopoldshafen, Germany. E-mail: Lars.Heinke@kit.edu
bKIT, Institute of Biological Interfaces (IBG-2), 76344 Eggenstein-Leopoldshafen, Germany
cKIT, Institute of Biological Interfaces 3 – Soft Matter Lab (IBG-3), 76344 Eggenstein-Leopoldshafen, Germany
dKIT, Institute of Toxicology and Genetics (ITG), 76344 Eggenstein-Leopoldshafen, Germany
eKIT, Institute of Organic Chemistry (IOC), 76131 Karlsruhe, Germany
First published on 8th May 2019
Abstract
A chiral photoswitchable nanoporous material with remote-controllable enantioselective adsorption capacity is presented. This metal–organic framework possesses both homochiral D-camphoric acid and light-responsive azobenzene moieties. Although the structure at the chiral moieties is unaffected, the trans–cis-azobenzene-photoisomerization changes the pore environment and, thus, switches the enantioselective adsorption behavior of the homochiral MOF.
Chiral structures are a central motif in all biological and many artificial molecules, and their (enantioselective) response is fundamental in medical, pharmaceutical and agricultural chemistry.1–4 Directing and switching the chirality are imperative to many chemical processes as well as for controlling the structure and functionality of numerous biological systems and molecular assemblies.5–8 In contrast to the chirality control by chemical stimuli,5,8 use of light as a stimulus can enable very fast switching over long distances with very high spatial resolution. Thus, dynamic remote control over the chirality by reversible photo-isomerization between different molecular enantiomers presents a major aim in chemistry. Recently, the control over the chirality of metal complexes by unidirectional molecular rotary motors based on overcrowded alkenes has been presented9 as well as chiral diarylethene compounds with photoswitchable chirality transfer.10 The control over the enantioselectivity in catalysis by light has also been demonstrated using molecular rotors based on overcrowded alkenes11 as well as with diarylethene.12 The chirality of liquid crystals can also be controlled by chiral molecular photoswitches.13 On the other hand, solid materials with remote-controllable chiral properties or porous materials with switchable enantioselective adsorption or separation properties have not yet been presented.
Metal–organic frameworks, MOFs, are a sub-class of crystalline nanoporous materials, composed of metal nodes connected by organic linker molecules.14,15 By using chiral linker molecules, chiral MOFs can be prepared.16 Due to their large variety and their ability to tune their properties by designing and rationally modifying the components, MOF materials are intensively investigated with the aim of efficient separation of achiral17 and chiral18 molecules. MOFs with remote-controllable properties can be prepared by incorporating photoswitchable molecules like azobenzene,19–22 spiropyran23,24 or diarylethene.25–27 Photoswitchable MOFs with azobenzene pendant groups22 have been used to dynamically control the color,28,29 adsorption,30–32 diffusion and release properties of the guest molecules33,34 and the membrane separation performance.35,36
Here, a nanoporous material, that is a pillared-layer MOF thin film, is presented which possesses both photoswitchable and chiral properties, Fig. 1. While the pillar linker, AzoBiPyB (= (E)-2-(phenyldiazenyl)-1,4-bis(4-pyridyl)benzene), possesses photoswitchable azobenzene side groups, the layer linker, D-camphoric acid (DCam), is homochiral. As a result, each pore of the pillared-layer Cu2(DCam)2(AzoBiPyB) MOF comprises two chiral centers and one photoswitchable moiety. The MOF synthesis is performed in a controlled layer-by-layer fashion resulting in thin surface-mounted MOF films, referred to as SURMOFs.37 These thin films enable the homogeneous irradiation of the entire sample, UV-vis and CD transmission spectroscopy as well as loading experiments with a quartz crystal microbalance (QCM). By performing uptake experiments with enantiopure probe molecules, (S)- or (R)-phenylethanol, we show that the nanoporous homochiral azobenzene-MOF thin film can be photoswitched between enantioselective and unselective forms.
The Cu2(DCam)2(AzoBiPyB) SURMOF is grown in a layer-by-layer (lbl) fashion by alternatively exposing the substrate to both synthesis solutions, ethanolic copper(II) acetate and the ethanolic linker solution of D-camphoric acid and AzoBiPyB. Between each immersion step, the samples were cleaned with pure ethanol. More details on the SURMOF synthesis can be found in previous publications.37,39 X-ray diffractograms, Fig. 2a, show that the sample is crystalline and has the targeted structure. The film is grown in the [001] direction, with the AzoBiPyB SURMOF linker perpendicular to the surface, as pictured in Fig. 1.
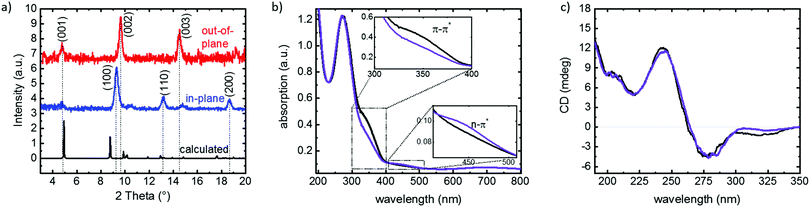 | ||
| Fig. 2 Cu2(DCam)2(AzoBiPyB) SURMOF characterization. (a) X-ray diffractograms of the sample in the out-of-plane (red) and in-plane (blue) geometry. The calculated diffractogram is shown in black. The crystal planes of the diffraction peaks are labelled. (b) UV-vis spectra of the sample in the thermodynamically stable trans state (black) and upon irradiation with UV light, resulting in the cis state (violet). The π–π* and n–π* bands of azobenzene are shown in the insets. The large absorption band at about 270 nm results from the ligand-to-metal charge transfer at the Cu node.38 (c) CD spectra of the sample in the trans (black) and cis (violet) state. | ||
UV-vis spectra, Fig. 2b, show that the absorption band at 320 nm, which is correlated with the azobenzene π–π* transition, decreases upon UV irradiation. The π–π* band increases to its initial value upon irradiation with blue light (455 nm) or by thermal relaxation. The absorption band at 450 nm, correlated with the azobenzene n–π* transition, increases upon UV irradiation and decreases again upon blue light irradiation or thermal relaxation. Comparison with the switching of azobenzene in solution40 or of the AzoBiPyB linker molecule in solution41 is a clear indication for UV-light-induced isomerization from the thermodynamically stable trans state to the excited cis state, while blue light as well as thermal relaxation results in cis-to-trans isomerization. This is in agreement with previous investigations of photoswitchable SURMOFs with the AzoBiPyB-linker.35,42 The time constant for thermal cis-to-trans isomerization was determined to be about 3 weeks at room temperature and approximately 10 h at 50 °C,42 which is much longer than the running time of the enantioselective adsorption experiments.
Infrared vibrational spectroscopy, ESI,† Fig. S1, and comparison with ref. 43 and 44 also verify the trans–cis isomerization. This shows that, upon UV irradiation, the maximum yield of cis azobenzene is approximately 60% (and 40% trans), referred to as the cis state. This value corresponds to the switching yield found in similar MOF structures.35,45 The maximum amount of trans azobenzene is 100%, obtained upon thermal relaxation. This is referred to as the trans state.
The chiral properties of the SURMOF are investigated by circular dichroism (CD) spectroscopy, Fig. 2c. The CD spectra are virtually identical to the CD spectra of a similar chiral MOF thin film with identical chiral D-camphoric acid linkers.46 Moreover, the chiral optical properties of the SURMOF with the azobenzene groups in the trans and in the cis state are essentially identical. Identical CD spectra for trans and cis were expected, since the photoswitchable groups are separated from the chiral D-camphoric acid centers.
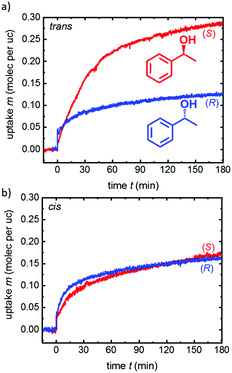
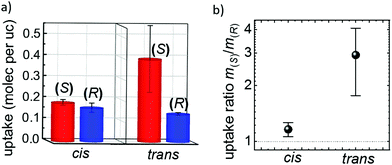
The enantiopure uptakes of (S)- and (R)-phenylethanol by the homochiral SURMOF in the trans and cis states recorded with a QCM47 are shown in Fig. 3. In the trans-SURMOF, Fig. 3a, the adsorption capacity for (S)-phenylethanol is significantly larger than the adsorption capacity for (R)-phenylethanol. The average of all uptake runs shows that the uptake of (S)-phenylethanol is approximately 2.9 ± 1.1 times as large as the uptake of (R)-phenylethanol. The enantioselective behavior dramatically changes upon UV irradiation of the Cu2(DCam)2(AzoBiPyB) SURMOF sample. In the cis state, the adsorption capacities of (S)- and (R)-phenylethanol are very similar and no significant enantioselective behavior was found. On average, the uptake by the cis-SURMOF of (S)-phenylethanol is approximately 1.16 ± 0.10 times the uptake of (R)-phenylethanol. The summary of the enantioselective behavior is shown in Fig. 4. The theoretical enantiomeric excesses ee of (S)- versus (R)-phenylethanol can be calculated from the enantiopure uptakes, where the interaction between the different enantiomers is neglected, corresponding to the ee at very low concentrations. As a result, while the ee in the cis state is only 7 ± 4%, the ee in the trans state is 49 ± 15%.
The enantioselectivity switching effect can be understood on a qualitative level. In the trans state, the nonpolar azobenzene moieties have only minor interaction with the alcohol guest molecules, as found in a previous publication.48 The interaction with other parts of the framework prevails, here with the chiral DCam linker molecules. In agreement with (R)/(S)-(2)-butanol in camphorate-MOFs,49 we assume the formation of H-bonds and dipole–dipole interactions between the guest's OH-group and the host's carboxylate group in proximity to the chiral MOF centers. This causes the observed large enantioselective adsorption of the nanoporous material. In contrast, the attractive interaction between the polar (cis) azobenzene and the polar guest molecules is significantly increased in the cis SURMOF, based on dipole–dipole interaction and the formation of H-bonds.48,50 So, the guest binds at the azobenzene moiety and not at the chiral center. Thus, the enantioselective adsorption properties are minute. The fact that the enantioselective response of the MOF material depends not only on the chiral center but also on the pore environment was previously found, where a significant impact of the pore size on the enantioselective adsorption was demonstrated.51 It has to be stated that although large efforts have been made, quantum-chemical calculation using both periodic and molecular density functional theory for the simulation of the observed switchable enantioselective adsorption behavior were not successful.
The enantioselective properties of a homochiral nanoporous MOF material significantly depend on the guest–host interaction, which is typically more complex than the simple three-point interaction.52 Since clear rules for determining the MOF material properties allowing efficient enantioselective separation of a given pair of chiral molecules, or vice versa, have not yet been presented, the research is often done by trial-and-error. It was found that the enantioselective response of a homochiral MOF material significantly depends on the pair of guest molecules.49,53–55 Here, complementary to the experiments with (R)- and (S)-phenylethanol, the enantiopure uptakes of less polar (R)- and (S)-2-octanol by the chiral SURMOF were investigated, Fig. S2 (ESI†); where there was no large difference found in both between the enantiomers and between the trans and cis forms of the host SURMOF.
In conclusion, a nanoporous thin film is presented which possesses both photoswitchable azobenzene and chiral camphoric acid moieties in the host framework. Uptake experiments with (S)- and (R)-phenylethanol as probe molecules show that the enantioselective adsorption behavior changes tremendously by the azobenzene photoisomerization – although the chiral centers are not modified. While the MOF thin film with azobenzene in the cis state shows only minute enantioselectivity, the MOF with the azobenzene in the trans state shows strong enantioselective behavior.
The switching of the enantioselective interaction can be extended to materials with chiral azobenzene moieties56,57 or photoswitches that can be switched between two chiral forms.9,11 Moreover, incorporating such photoswitches in MOF materials with even more complex pore structures and different chiral centers is possible, resulting in switching between different complex stereoselective interactions. We foresee that this concept helps realize efficient enantioselective separation via membranes58,59 that allow dynamic switching between racemic mixture and enantioenriched permeates.
We are grateful to the Volkswagenstiftung and the DFG (HE 7036/5) for financial support. We thank Tobias Schlöder and Wolfgang Wenzel (KIT) for their efforts and attempts to quantum-chemically simulate the observed enantioselective adsorption and for fruitful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. W. Green, J.-M. Lee, E.-J. Kim, D.-J. Lee and H.-S. Jung, Adv. Mater. Interfaces, 2016, 3, 1500411 CrossRef.
- E. Yashima, K. Maeda, H. Iida, Y. Furusho and K. Nagai, Chem. Rev., 2009, 109, 6102–6211 CrossRef CAS.
- L. D. Barron, Space Sci. Rev., 2008, 135, 187–201 CrossRef CAS.
- I. Agranat, H. Caner and A. Caldwell, Nat. Rev. Drug Discovery, 2002, 1, 753–768 CrossRef CAS PubMed.
- H. Miyake and H. Tsukube, Chem. Soc. Rev., 2012, 41, 6977–6991 RSC.
- D. Pijper and B. L. Feringa, Soft Matter, 2008, 4, 1349–1372 RSC.
- J. Crassous, Chem. Soc. Rev., 2009, 38, 830–845 RSC.
- R. M. Meudtner and S. Hecht, Angew. Chem., Int. Ed., 2008, 47, 4926–4930 CrossRef CAS PubMed.
- D. Zhao, T. van Leeuwen, J. Cheng and B. L. Feringa, Nat. Chem., 2016, 9, 250 CrossRef PubMed.
- C. Jurissek, F. Berger, F. Eisenreich, M. Kathan and S. Hecht, Angew. Chem., Int. Ed., 2019, 58, 1945–1949 CrossRef CAS PubMed.
- D. P. Zhao, T. M. Neubauer and B. L. Feringa, Nat. Commun., 2015, 6, 7 CrossRef.
- D. Sud, T. B. Norsten and N. R. Branda, Angew. Chem., Int. Ed., 2005, 44, 2019–2021 CrossRef CAS.
- Y. Wang and Q. Li, Adv. Mater., 2012, 24, 1926–1945 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kaskel, The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications, Wiley, 2016 Search PubMed.
- G. Nickerl, A. Henschel, R. Grunker, K. Gedrich and S. Kaskel, Chem. Ing. Tech., 2011, 83, 90–103 CrossRef CAS.
- N. Rangnekar, N. Mittal, B. Elyassi, J. Caro and M. Tsapatsis, Chem. Soc. Rev., 2015, 44, 7128–7154 RSC.
- T. Duerinck and J. F. M. Denayer, Chem. Eng. Sci., 2015, 124, 179–187 CrossRef CAS.
- C. L. Jones, A. J. Tansell and T. L. Easun, J. Mater. Chem. A, 2016, 4, 6714–6723 RSC.
- O. S. Bushuyev, T. Friscic and C. J. Barrett, CrystEngComm, 2016, 18, 7204–7211 RSC.
- S. Castellanos, F. Kapteijn and J. Gascon, CrystEngComm, 2016, 18, 4006–4012 RSC.
- A. B. Kanj, K. Müller and L. Heinke, Macromol. Rapid Commun., 2017, 38, 1700239 Search PubMed.
- S. Garg, H. Schwartz, M. Kozlowska, A. B. Kanj, K. Müller, W. Wenzel, U. Ruschewitz and L. Heinke, Angew. Chem., 2019, 58, 1193–1197 CrossRef CAS PubMed.
- H. A. Schwartz, S. Olthof, D. Schaniel, K. Meerholz and U. Ruschewitz, Inorg. Chem., 2017, 56, 13100–13110 CrossRef CAS PubMed.
- D. G. Patel, I. M. Walton, J. M. Cox, C. J. Gleason, D. R. Butzer and J. B. Benedict, Chem. Commun., 2014, 50, 2653–2656 RSC.
- I. M. Walton, J. M. Cox, J. A. Coppin, C. M. Linderman, D. G. Patel and J. B. Benedict, Chem. Commun., 2013, 49, 8012–8014 RSC.
- J. Park, D. Feng, S. Yuan and H.-C. Zhou, Angew. Chem., Int. Ed., 2015, 54, 430–435 CrossRef CAS PubMed.
- S. Bernt, M. Feyand, A. Modrow, J. Wack, J. Senker and N. Stock, Eur. J. Inorg. Chem., 2011, 5378–5383 CrossRef CAS.
- J. S. Caddy, T. B. Faust, I. M. Walton, J. M. Cox, J. B. Benedict, M. B. Solomon, P. D. Southon, C. J. Kepert and D. M. D'Alessandro, Aust. J. Chem., 2017, 70, 1171–1179 CrossRef CAS.
- A. Modrow, D. Zargarani, R. Herges and N. Stock, Dalton Trans., 2012, 41, 8690–8696 RSC.
- J. Park, D. Q. Yuan, K. T. Pham, J. R. Li, A. Yakovenko and H. C. Zhou, J. Am. Chem. Soc., 2012, 134, 99–102 CrossRef CAS PubMed.
- Y. Zhu and W. Zhang, Chem. Sci., 2014, 5, 4957–4961 RSC.
- J. Brown, B. L. Henderson, M. D. Kiesz, A. C. Whalley, W. Morris, S. Grunder, H. Deng, H. Furukawa, J. I. Zink, J. F. Stoddart and O. M. Yaghi, Chem. Sci., 2013, 4, 2858–2864 RSC.
- L. Heinke, M. Cakici, M. Dommaschk, S. Grosjean, R. Herges, S. Bräse and C. Wöll, ACS Nano, 2014, 8, 1463–1467 CrossRef CAS PubMed.
- Z. Wang, A. Knebel, S. Grosjean, D. Wagner, S. Bräse, C. Wöll, J. Caro and L. Heinke, Nat. Commun., 2016, 7, 13872 CrossRef CAS PubMed.
- K. Müller, A. Knebel, F. Zhao, D. Bléger, J. Caro and L. Heinke, Chem. – Eur. J., 2017, 23, 5434–5438 CrossRef PubMed.
- O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef CAS PubMed.
- Z. Gu, L. Heinke, C. Wöll, T. Neumann, W. Wenzel, Q. Li, K. Fink, O. D. Gordan and D. R. T. Zahn, Appl. Phys. Lett., 2015, 107, 183301 CrossRef.
- L. Heinke and C. Wöll, Adv. Mater., 2019, 1806324 CrossRef PubMed.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- Z. Wang, L. Heinke, J. Jelic, M. Cakici, M. Dommaschk, R. J. Maurer, H. Oberhofer, S. Grosjean, R. Herges, S. Bräse, K. Reuter and C. Wöll, Phys. Chem. Chem. Phys., 2015, 17, 14582–14587 RSC.
- X. Yu, Z. Wang, M. Buchholz, N. Fullgrabe, S. Grosjean, F. Bebensee, S. Bräse, C. Wöll and L. Heinke, Phys. Chem. Chem. Phys., 2015, 17, 22721–22725 RSC.
- D. Hermann, H. Emerich, R. Lepski, D. Schaniel and U. Ruschewitz, Inorg. Chem., 2013, 52, 2744–2749 CrossRef CAS PubMed.
- L. Duarte, R. Fausto and I. Reva, Phys. Chem. Chem. Phys., 2014, 16, 16919–16930 RSC.
- Z. B. Wang, K. Müller, M. Valasek, S. Grosjean, S. Bräse, C. Wöll, M. Mayor and L. Heinke, J. Phys. Chem. C, 2018, 122, 19044–19050 CrossRef CAS.
- Z.-G. Gu, J. Bürck, A. Bihlmeier, J. Liu, O. Shekhah, P. G. Weidler, C. Azucena, Z. Wang, S. Heissler, H. Gliemann, W. Klopper, A. S. Ulrich and C. Wöll, Chem. – Eur. J., 2014, 20, 9879–9882 CrossRef CAS PubMed.
- D. Johannsmann, The Quartz Crystal Microbalance in Soft Matter Research, Springer, 2015 Search PubMed.
- Z. Wang, S. Grosjean, S. Bräse and L. Heinke, ChemPhysChem, 2015, 16, 3779–3783 CrossRef CAS PubMed.
- Y. A. Satska, E. A. Mikhalyova, Z. V. Chernenko, S. V. Kolotilov, M. Zeller, I. V. Komarov, A. V. Tymtsunik, A. Tolmachev, K. S. Gavrilenko and A. W. Addison, RSC Adv., 2016, 6, 93707–93714 RSC.
- K. Müller, J. Helfferich, F. L. Zhao, R. Verma, A. B. Kanj, V. Meded, D. Bléger, W. Wenzel and L. Heinke, Adv. Mater., 2018, 30, 1706551 CrossRef PubMed.
- Z. Gu, S. Grosjean, S. Bräse, C. Wöll and L. Heinke, Chem. Commun., 2015, 51, 8998–9001 RSC.
- A. Berthod, Chiral Recognition in Separation Methods: Mechanisms and Applications, Springer Berlin Heidelberg, 2010 Search PubMed.
- F. Song, C. Wang, J. M. Falkowski, L. Ma and W. Lin, J. Am. Chem. Soc., 2010, 132, 15390–15398 CrossRef CAS PubMed.
- J. Zhang, Z. J. Li, W. Gong, X. Han, Y. Liu and Y. Cui, Inorg. Chem., 2016, 55, 7229–7232 CrossRef CAS PubMed.
- S. A. Boer, Y. Nolvachai, C. Kulsing, L. J. McCormick, C. S. Hawes, P. J. Marriott and D. R. Turner, Chem. – Eur. J., 2014, 20, 11308–11312 CrossRef CAS PubMed.
- A. Painelli, F. Terenziani, L. Angiolini, T. Benelli and L. Giorgini, Chem. – Eur. J., 2005, 11, 6053–6063 CrossRef CAS PubMed.
- B. L. Feringa, R. A. van Delden, N. Koumura and E. M. Geertsema, Chem. Rev., 2000, 100, 1789–1816 CrossRef CAS PubMed.
- Z. X. Kang, M. Xue, L. L. Fan, J. Y. Ding, L. J. Guo, L. X. Gao and S. L. Qiu, Chem. Commun., 2013, 49, 10569–10571 RSC.
- K. Huang, X. Dong, R. Ren and W. Jin, AIChE J., 2013, 59, 4364–4372 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and infrared spectra. See DOI: 10.1039/c9cc02849h |
| This journal is © The Royal Society of Chemistry 2019 |