 Open Access Article
Open Access ArticlePhoto-controlled delivery of a potent analogue of doxorubicin†
Patrick S.
Dupart
ab,
Koushambi
Mitra
 ab,
Charles E.
Lyons
b and
Matthew C. T.
Hartman
ab,
Charles E.
Lyons
b and
Matthew C. T.
Hartman
 *ab
*ab
aDepartment of Chemistry, Virginia Commonwealth University, 1001 W Main St, Richmond, 23284, VA, USA. E-mail: mchartman@vcu.edu
bMassey Cancer Center, Virginia Commonwealth University, 401 College St, Richmond, 23219, Virginia, USA. Tel: +1-804-628-4095
First published on 25th April 2019
Abstract
Highly cytotoxic agents have found an important niche in targeted anticancer therapy. Here we develop a new light release strategy for the targeting of one of these agents, 2-pyrrolinodoxorubicin, showing dramatic enhancements in toxicity with light and single digit nM potency.
One of the major contributing factors for the failure of promising anticancer drugs in the clinic is off-target toxicity.1,2 This problem is particularly acute when one considers highly potent cytotoxins as potential anticancer therapeutics.2 While they are potent anticancer agents, the high toxicity typically leads to a very narrow therapeutic window that prevents clinical use. Instead, research has focused on developing novel methods using antibodies to target these molecules directly to the tumor itself.3–6
A promising alternative strategy for directing the release of highly potent cytotoxins involves the use of light. To date, photodynamic therapy (PDT) has proven a popular and effective strategy for treatment of cancer with light.7–9 PDT relies on an administered photosensitizer that is activated using wavelengths of light between 650–800 nm. Once activated, the photosensitizer (PS) creates singlet oxygen, which is cytotoxic to cancerous cells. However, the deeper regions of tumors are typically hypoxic, making photodynamic therapy ineffective for larger tumors. Moreover, the short-lived nature of singlet oxygen prevents its diffusion into deeper regions of the tumor where light cannot penetrate.10
A more recent alternative to PDT is photoactivated chemotherapy where a standard cancer chemotherapeutic is converted into a light-activatable form.11,12 A common strategy is to attach the molecule to a photocage,13 a releasable group that blocks the anticancer agent's activity until illumination. We and others have pursued this strategy by caging conventional anticancer agents such as doxorubicin, cisplatin, etc.12,14–21 Each of these advances has focused on delivery of anticancer agents with moderate cytotoxicity. Release of a more potent drug should enable deeper tissue activation as less efficient release should still lead to the cytotoxic effect.
Here we describe the photoactivation of one of these highly potent agents, 2-pyrrolino doxorubicin (2P-Dox)22,23 (Fig. 1a). 2P-Dox is a derivative of the standard cancer chemotherapeutic doxorubicin (Dox) which is prescribed for the treatment of a variety of cancers including ovarian, breast, and lung cancer, as well as leukemia.24 2P-Dox is 100–1000 fold more potent than Dox in vitro against a number of Dox resistant cell lines, and has IC50 values in the low nM to high pM range.25–28 The extreme potency of 2P-Dox is a result of its ability to form an aminal adduct with an amino group of a guanine base in close vicinity to its binding site in DNA.27 2P-Dox is also not a substrate of the P-gp pump which is the key factor for resistance of Dox.29 Research efforts have focused on targeting 2P-Dox through antibody or peptide conjugation,22,30,31 but here we describe the synthesis and analysis of a photoactivatable version of 2P-Dox.
We have based our strategy on the di-acetoxy prodrug of 2P-Dox.25,27 The acetates on this prodrug are cleaved intracellularly by esterases releasing the latent aldehyde which cyclizes to form 2P-Dox (Fig. 1b). We reasoned that a suitable photo-caging group for the latent aldehyde13 would lead to a light-activatable 2P-Dox (1, Fig. 1b).
Kantevari et al. previously utilized a bis(4,5-dimethoxy-2-nitrophenyl)ethylene glycol for the photorelease of various aldehydes and ketones through acetal protection.32 We have chosen to use a modified version of this strategy for our drug delivery. To prepare compound 1 (Scheme 1), we started by a chlorination of commercially available nitroveratryl alcohol (2), which was then reacted with triphenyl phosphine to generate phosphonium salt 3. The salt was then reacted with formaldehyde under Wittig reaction conditions to give a styrene which was dihydroxylated to give diol 4. The diol was acetal protected with commercially available 3-cyanopropionaldehyde dimethyl acetal (5) to give diastereomeric acetals, and the nitrile group was reduced with DIBAL-H33 to give aldehyde 6 which was coupled to doxorubicin under reductive amination conditions to yield 1. The overall yield was 41%. We also prepared a control compound, 7, formed by the reductive amination of aldehyde 6 and cyclohexylamine.
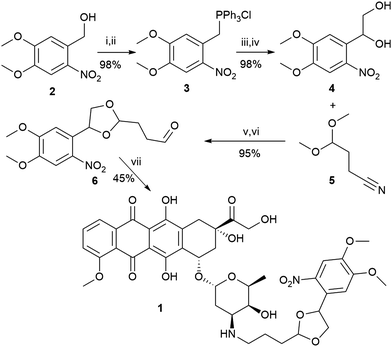 | ||
| Scheme 1 (i) SOCl2; (ii) PPh3/toluene; (iii) formaldehyde/water; (iv) OsO4/NMO/CH2Cl2/H2O; (v) PPTS/benzene; (vi) DIBAL-H/CH2Cl2; (vii) NaCNBH3/H2O. | ||
With 1 in hand, we investigated the rates of release of 2P-Dox under UV illumination via thin layer chromatography (Fig. 2). Cleavage begins to take place after 2 min with 88% cleavage after 60 min irradiation (Fig. 2a and b). A clean MS showing 2P-Dox was observed after 60 min (Fig. 2c). No cleavage was observed in the dark (not shown). HPLC traces of the reaction before and after illumination were consistent with the TLC results (Fig. S1, ESI†).
Once we determined that light had no effect on cytotoxicity (Fig. S2a, ESI†) we proceeded with cellular studies to demonstrate that the 2P-Dox released led to the expected enhancement in cytotoxicity. We performed the cellular viability assay in three human cancer cell lines: MCF-7 (breast), A2780 (ovarian) and A2780ADR (doxorubicin resistant ovarian) using the CellTiter-Blue method to evaluate the light-induced effects of compound 1 as well control compound 7 (Table 1, Fig. 3 and Fig. S2–S5, ESI†). We observed excellent photo-induced toxicity of compound 1 with impressive 327–750 fold increase over dark toxicity. 1 exhibited remarkably low IC50 values ranging from 0.1–50 nM in presence of light (380 nm, 30 min); these values were comparable to that obtained for 2P-Dox alone. The control compound 7 showed no toxicity; demonstrating that the cytotoxic effects were completely due to released drug (Fig. S5, ESI†). In accord with previously reported observations,25–27 we found that 2P-Dox displayed higher potency than doxorubicin and also retained its activity in the doxorubicin-resistant cell line A2780ADR, where it was 330-fold more active than doxorubicin alone in A2780ADR cells.
| MCF-7 | A2780 | A2780ADR | ||||
|---|---|---|---|---|---|---|
| (+) light | (−) light | (+) light | (−) light | (+) light | (−) light | |
| 1 | 0.05 ± 0.01 | 18.7 ± 2.4 | 0.002 ± 0.001 | 1.5 ± 0.6 | 0.003 ± 0.001 | 0.98 ± 0.26 |
| Dox | 1.3 ± 0.2 | 1.4 ± 0.1 | 0.076 ± 0.016 | 0.095 ± 0.021 | 0.99 ± 0.25 | 1.3 ± 0.3 |
| 2P-Dox | 0.010 ± 0.007 | 0.013 ± 0.005 | n.d. | 0.0017 ± 0.0004 | n.d. | 0.002 ± 0.001 |
| 7 | >50 | >50 | >50 | >50 | >50 | >50 |
We next exploited the fluorescent properties of doxorubicin and compound 1 to quantify the cellular uptake in MCF-7 cell lines using flow cytometry (Fig. S6, ESI†). The obtained data clearly indicates that compound 1 showed higher cellular uptake than doxorubicin. Several studies have shown that N-alkylation or O-alkylation of DOX with lipophilic moieties can improve both the amount and rate of cell uptake,34–36,41 and we expect a similar phenomenon is operating here. The enhanced uptake also likely contributes to the improved cytotoxicity of compound 1 relative to Dox. To understand the sub-cellular localization, we carried out confocal microscopic studies. The images revealed the nuclear co-localization of compound 1 and doxorubicin (Fig. 3c). Taken together, our light-releasable compound 1 mirrors the activity of the parent drug 2P-Dox, yet has significantly reduced activity in the dark.
Here we have shown for the first time that we can generate 2P-Dox in a light dependent manner. The large enhancements in activity suggest that this approach will have an effective therapeutic window. Although UV light is poorly tissue penetrating, one could envision the use of alternate protecting groups that can be released with longer wavelengths of light that are more penetrant.37–40 The high potency of 2P-Dox will further improve tissue penetration as even poorly illuminated deep tissues will contain enough released 2P-Dox for the cytotoxic effect. More broadly, this work opens up new opportunities for repurposing of highly potent cytotoxins for effective cancer chemotherapy.
M. C. T. H. acknowledges support of this work by the NIH (CA167582). The mass spectral analyses were done with Massey Cancer Center Proteomics Resources supported by CCSG grant NCI 5P30CA16059-35. P. S. D. acknowledges the Altria Corporation for a graduate fellowship. K. M.'s postdoctoral fellowship was supported by the Virginia Commonwealth Health Research Board (236-03-16).
Conflicts of interest
M. C. T. H. is a part of a company, LightSwitch Bio, that is developing related technologies for commercial application.References
- A. L. Hopkins, Nat. Chem. Biol., 2008, 4, 682–690 CrossRef CAS PubMed.
- S. Wollowitz, Drug Dev. Res., 2010, 71, 420–428 CrossRef CAS.
- I. Sassoon and V. Blanc, Antibody-drug conjugate (ADC) clinical pipeline: a review, 2013 Search PubMed.
- J. M. Lambert and A. Berkenblit, Annu. Rev. Med., 2018, 69, 191–207 CrossRef CAS PubMed.
- A. Beck, L. Goetsch, C. Dumontet and N. Corvà, Nat. Rev. Drug Discovery, 2017, 16, 315 CrossRef CAS PubMed.
- R. R. Nani, A. P. Gorka, T. Nagaya, T. Yamamoto, J. Ivanic, H. Kobayashi and M. J. Schnermann, ACS Cent. Sci., 2017, 3, 329–337 CrossRef CAS PubMed.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380 CrossRef CAS PubMed.
- J. Zhang, C. Jiang, J. P. F. Longo, R. B. Azevedo, H. Zhang and L. A. Muehlmann, Acta Pharm. Sin. B, 2018, 8, 137–146 CrossRef PubMed.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19–33 RSC.
- T. Maisch, J. Baier, B. Franz, M. Maier, M. Landthaler, R.-M. Szeimies and W. Bäumler, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7223–7228 CrossRef CAS PubMed.
- N. J. Farrer, L. Salassa and P. J. Sadler, Dalton Trans., 2009, 10690–10701 RSC.
- F. Reeßing and W. Szymanski, Curr. Med. Chem., 2018, 24, 4905–4950 CrossRef PubMed.
- P. Klán, T. Solomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2012, 113, 119–191 CrossRef PubMed.
- S. Ibsen, E. Zahavy, W. Wrasdilo, M. Berns, M. Chan and S. Esener, Pharm. Res., 2010, 27, 1848–1860 CrossRef CAS PubMed.
- S. K. Choi, T. Thomas, M. H. Li, A. Kotlyar, A. Desai and J. R. Baker, Chem. Commun., 2010, 46, 2632–2634 RSC.
- P. T. Wong, S. Tang, J. Cannon, D. Chen, R. Sun, J. Lee, J. Phan, K. Tao, K. Sun, B. Chen, J. R. Baker and S. K. Choi, Bioconjugate Chem., 2017, 28, 3016–3028 CrossRef CAS PubMed.
- M. M. Dcona, J. E. Sheldon, D. Mitra and M. C. Hartman, Bioorg. Med. Chem. Lett., 2017, 27, 466–469 CrossRef CAS PubMed.
- M. M. Dcona, D. Mitra, R. W. Goehe, D. A. Gewirtz, D. A. Lebman and M. C. Hartman, Chem. Commun., 2012, 48, 4755–4757 RSC.
- M. Noguchi, M. Skwarczynski, H. Prakash, S. Hirota, T. Kimura, Y. Hayashi and Y. Kiso, Bioorg. Med. Chem., 2008, 16, 5389–5397 CrossRef CAS PubMed.
- K. Mitra, S. Gautam, P. Kondaiah and A. R. Chakravarty, Angew. Chem., Int. Ed., 2015, 54, 13989–13993 CrossRef CAS PubMed.
- K. Mitra, C. E. Lyons and M. C. Hartman, Angew. Chem., Int. Ed., 2018, 57, 10263–10267 CrossRef CAS PubMed.
- A. Nagy, A. V. Schally, G. Halmos, P. Armatis, R. Z. Cai, V. Csernus, M. Kovacs, M. Koppan, K. Szepeshazi and Z. Kahan, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 1794–1799 CrossRef CAS.
- A. Nagy, P. Armatis, R. Z. Cai, K. Szepeshazi, G. Halmos and A. V. Schally, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 652–656 CrossRef CAS.
- R. C. Young, R. F. Ozols and C. E. Myers, N. Engl. J. Med., 1981, 305, 139–153 CrossRef CAS PubMed.
- A. Cherif and D. Farquhar, J. Med. Chem., 1992, 35, 3208–3214 CrossRef CAS PubMed.
- D. Farquhar, A. Cherif, E. Bakina and J. A. Nelson, J. Med. Chem., 1998, 41, 965–972 CrossRef CAS PubMed.
- L. A. Zwelling, E. Altschuler, A. Cherif and D. Farquhar, Cancer Res., 1991, 51, 6704–6707 CAS.
- A. Nagy, P. Armatis and A. V. Schally, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 2464–2469 CrossRef CAS.
- C. Castex, P. Merida, E. Blanc, P. Clair, A. R. Rees and J. Temsamani, Anti-Cancer Drugs, 2004, 15, 609–617 CrossRef CAS.
- A. Nagy, A. V. Schally, P. Armatis, K. Szepeshazi, G. Halmos, M. Kovacs, M. Zarandi, K. Groot, M. Miyazaki and A. Jungwirth, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 7269–7273 CrossRef CAS.
- S. C. Jeffrey, M. T. Nguyen, J. B. Andreyka, D. L. Meyer, S. O. Doronina and P. D. Senter, Bioorg. Med. Chem. Lett., 2006, 16, 358–362 CrossRef CAS PubMed.
- S. Kantevari, C. V. Narasimhaji and H. B. Mereyala, Tetrahedron, 2005, 61, 5849–5854 CrossRef CAS.
- L. A. Paquette, D. Backhaus, R. Braun, T. L. Underiner and K. Fuchs, J. Am. Chem. Soc., 1997, 119, 9662–9671 CrossRef CAS.
- L. Lothstein, P. J. Rodrigues, T. W. Sweatman and M. Israel, Anticancer Drugs, 1998, 9, 58–66 CrossRef CAS.
- M. Israel, T. W. Sweatman, R. Seshadri and Y. Koseki, Cancer Chemother. Pharmacol., 1989, 25, 177–183 CrossRef CAS.
- M. Israel, R. Seshadri, Y. Koseki, T. W. Sweatman and J. M. Idriss, Cancer Treat. Rev., 1987, 14, 163–167 CrossRef CAS PubMed.
- J. Jagdeo, E. Austin, A. Mamalis, C. Wong, D. Ho and D. M. Siegel, Lasers Surg. Med., 2018, 50, 613–628 CrossRef PubMed.
- D. Barolet, Semin. Cutaneous Med. Surg., 2008, 27, 227–238 CrossRef CAS PubMed.
- T. A. Shell and D. S. Lawrence, Acc. Chem. Res., 2015, 48, 2866–2874 CrossRef CAS PubMed.
- A. P. Gorka, R. R. Nani, J. Zhu, S. Mackem and M. J. Schnermann, J. Am. Chem. Soc., 2014, 136, 14153–14159 CrossRef CAS PubMed.
- L. Lothstein, H. M. Wright, T. W. Sweatman and M. Israel, Oncol. Res., 1992, 4, 341–347 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc02050k |
| This journal is © The Royal Society of Chemistry 2019 |



