Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of enantioenriched alkanol with stochastic distribution of enantiomers in the absolute asymmetric synthesis under heterogeneous solid–vapor phase conditions†
Yoshiyasu
Kaimori
,
Yui
Hiyoshi
,
Tsuneomi
Kawasaki
 ,
Arimasa
Matsumoto‡
,
Arimasa
Matsumoto‡
 and
Kenso
Soai
and
Kenso
Soai
 *
*
Department of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo, 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp
First published on 4th April 2019
Abstract
Among several theories proposed for the origin of homochirality, absolute asymmetric synthesis is unique because it produces chiral compounds without the intervention of any chiral factor. Here we report on the kinetically controlled heterogeneous solid–vapor phase absolute asymmetric synthesis in conjunction with asymmetric autocatalysis with amplification of chirality. Each reaction, carried out in a test tube, between achiral powder crystals of pyrimidine-5-carbaldehyde and the vapor of diisopropylzinc, is controlled kinetically to afford either (S)- or (R)-pyrimidyl alkanol.
The origin of biological homochirality such as that seen in L-amino acids and D-sugars has been a long-standing subject of considerable attention.1 Several theories have been proposed to explain the origins of the chirality of organic compounds, including circularly polarized light, chiral inorganic minerals, chiral crystals composed of achiral organic compounds, enantiotopic surfaces of achiral inorganic and organic crystals, chiral metal surfaces, spontaneous crystallization, and absolute asymmetric synthesis. However, in most cases, the enantiomeric excess (ee) of the products has been very low to moderate. Therefore, the mechanism of amplification of chirality is required to reach homochirality2 including self-disproportionation of enantiomers.3
Among the theories proposed to explain the origins of homochirality, absolute asymmetric synthesis is particularly important because unlike other mechanisms, it does not require any chiral factor. Mislow proposed a new definition of absolute asymmetric synthesis as “asymmetric synthesis without the intervention of any chiral factor.”1a It is widely accepted that organic reactions without the intervention of any chiral factor always give equal amounts of two enantiomers; i.e., racemate.
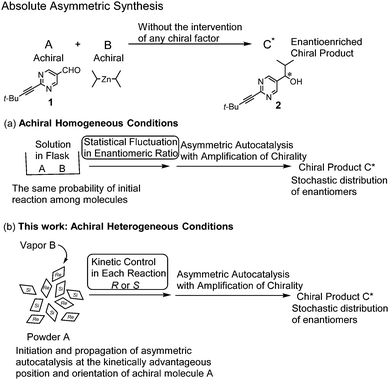
We have been studying asymmetric autocatalysis with significant amplification of ee. 5-Pyrimidyl alkanol acts as an asymmetric autocatalyst with amplification of ee in the enantioselective addition of diisopropylzinc (i-Pr2Zn) to pyrimidine-5-carbaldehyde 1.4–6 Starting from an asymmetric autocatalyst with as low as ca. 0.00005% ee, three consecutive asymmetric autocatalysis reactions afforded 5-pyrimidyl alkanol 2 with near enantiopurity (>99.5% ee) and multiplication of the amount by ca. 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times.4c Moreover, it was found that absolute asymmetric synthesis—i.e., the reaction between pyrimidine-5-carbaldehyde and i-Pr2Zn in solution, without the intervention of any chiral factor in combination, with asymmetric autocatalysis with amplification of ee—afforded an enantioenriched chiral product (Scheme 1a).7,8 The origin of chirality in these absolute asymmetric syntheses under homogeneous conditions, is considered to be based on the statistical fluctuations of enantiomeric ratio(s) of product formed from achiral reactants in the early stage of the reaction. Statistical enantiomeric fluctuations of racemates formed from achiral reactants have been estimated mathematically.1a,9 The process requires the initial homogeneous solution of substrates, which requires a substantial amount of solvent.
000 times.4c Moreover, it was found that absolute asymmetric synthesis—i.e., the reaction between pyrimidine-5-carbaldehyde and i-Pr2Zn in solution, without the intervention of any chiral factor in combination, with asymmetric autocatalysis with amplification of ee—afforded an enantioenriched chiral product (Scheme 1a).7,8 The origin of chirality in these absolute asymmetric syntheses under homogeneous conditions, is considered to be based on the statistical fluctuations of enantiomeric ratio(s) of product formed from achiral reactants in the early stage of the reaction. Statistical enantiomeric fluctuations of racemates formed from achiral reactants have been estimated mathematically.1a,9 The process requires the initial homogeneous solution of substrates, which requires a substantial amount of solvent.
 | ||
| Scheme 1 Absolute asymmetric synthesis initiated under (a) homogeneous and (b) heterogeneous conditions in conjunction with asymmetric autocatalysis with amplification of chirality. | ||
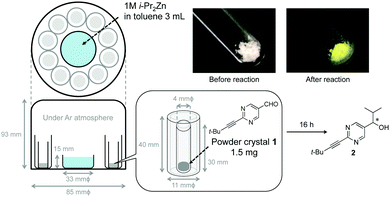
In sharp contrast, under heterogeneous conditions of the random orientation of powder crystals, the location and orientation of the reactant differ. There should be a few reactants with a kinetically advantageous position and orientation for the initiation and propagation of asymmetric autocatalysis (Scheme 1b). Here we report on the absolute asymmetric synthesis under a heterogeneous random surface of solid–vapor phase conditions in conjunction with asymmetric autocatalysis by exposing achiral powder crystals of pyrimidine-5-carbaldehyde 1 to i-Pr2Zn vapor (achiral crystal structure P21/n) (Scheme 2).
 | ||
| Scheme 2 Kinetic heterogeneous absolute asymmetric synthesis in conjunction with asymmetric autocatalysis between powder crystals of pyrimidine-5-carbaldehyde 1 and diisopropylzinc vapor. | ||
The present conditions of absolute asymmetric synthesis differ from those of our previously reported liquid phase conditions7 in the following ways. (i) A powder crystal of pyrimidine-5-carbaldehyde acts as a heterogeneous reactant. (ii) The surfaces of the powder pyrimidine-5-carbaldehyde are oriented in a randomly fixed manner. (iii) The orientations of the Re and Si faces of the carbaldehyde are random and fixed, at least in the initial stage of the reaction. (iv) Under the heterogeneous conditions described above, it is conceivable that the reaction is initiated at only a few points on the randomly orientated surface of the aldehyde.
Solid–vapor phase absolute asymmetric synthesis was performed by exposing powder crystals of pyrimidine-5-carbaldehyde 1 to i-Pr2Zn vapor (Scheme 2). To perform the solid–vapor phase reaction between the sublimed powder crystals of 1 and i-Pr2Zn vapor, 1 and i-Pr2Zn were placed separately in an airtight container (desiccator) (figure in Table 1). One set of reactions was performed with 10 or 11 samples of carbaldehyde 1 powder. The results are shown in Table 1 and summarized in Fig. 1. In the first set of reactions, carried out in the same container, enantioenriched (R)- and (S)-pyrimidyl alkanols 2 were obtained; (S)-alkanol 2 was formed five times, and (R)-alkanol 2 was formed six times. To examine the frequency of the absolute configurations of the formed alkanol 2, 129 reactions were then run under the same conditions. In total, (R)-alkanol 2 was formed 61 times, and (S)-alkanol 2 was formed 58 times (10 times the formation of 2 with <0.5% ee was assigned as below the detection level (BDL)). Although the ee values of alkanol 2 varied, it should be mentioned that these values could be amplified to >99.5% ee by the subsequent consecutive asymmetric autocatalysis using the product alkanol 2 as the asymmetric autocatalyst.4c
| Runa | Molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
ee/% and config. of 2c | Runa | Molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
ee/% and config. of 2c |
|---|---|---|---|---|---|
| Series A | Series B | ||||
| Series C–L (C23–L129): see Table S1 in ESI.a In the series A–L, each set of reactions was run simultaneously in the same desiccator. Each glass tube was used in only one reaction.b Determined using GC.c Determined using supercritical fluid chromatography analysis on a chiral stationary phase.d Under homogeneous conditions as previously reported (ref. 4c), further three consecutive asymmetric autocatalysis by using obtained 2 as the initial autocatalyst, afford highly enantioenriched alkanol 2. | |||||
| A1 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
0.6 (S) | B12 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
1.5 (R) (>99.5)d |
| A2 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
11.6 (S) | B13 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
5.2 (R) |
| A3 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
3.7 (R) | B14 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
1.2 (S) (>99.5)d |
| A4 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
5.0 (R) | B15 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
3.0 (S) |
| A5 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
6.8 (S) | B16 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
13.8 (R) |
| A6 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
5.3 (R) | B17 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
5.1 (R) |
| A7 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
11.8 (S) | B18 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
14.5 (S) |
| A8 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
1.6 (R) | B19 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
2.9 (R) |
| A9 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
16.9 (S) | B20 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
2.6 (S) |
| A10 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
4.7 (R) | B21 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
6.3 (S) |
| A11 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
3.3 (R) | B22 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
2.5 (S) |
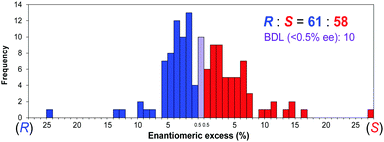 | ||
| Fig. 1 Relationship between the frequency, absolute configurations, and enantiomeric excesses of pyrimidyl alkanol 2. | ||
To analyze the above results based on statistical theories, we examined the frequency ratio equality of the (R)- and (S)-alkanols 2 generated. We calculated the Pearson chi-squared statistic for goodness of fit (χGF2) (Table S2, ESI†). Considering the observed frequencies (61 times (R)-2 and 58 times (S)-2) and theoretical frequency (59.5 times (R)- and (S)-2), χGF2 is sufficiently close to zero (χGF2 = 0.07563, p = 0.78). Accordingly, no deviation of the sense of absolute configurations was observed in these experimental results.
To examine the influence of the absolute configuration of the neighboring (isopropylzinc alkoxide of) alkanol 2, we calculated the chi-squared statistic for independence (χI2). The absolute configurations of one alkanol 2 and its right-hand (clockwise) neighboring alkanol 2 were counted in a contingency table (Table S3, ESI†). According to the contingency table, the value of χI2 is sufficiently close to zero (χI2 = 0.07196, p = 0.79). Thus, it was concluded that there is no significant correlation of the absolute configuration between neighboring (isopropylzinc alkoxide of) alkanols 2. According to chi-squared tests, the ratio of produced (R)- and (S)-alkanols 2 is nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Statistical analysis revealed here that the formation of 61 times (R)-2 and 58 times (S)-2 under solid–vapor phase reaction of pyrimidine-5-carbaldehyde 1 and i-Pr2Zn exhibits statistical distribution and satisfies one of the necessary conditions of spontaneous absolute asymmetric synthesis as defined by Mislow.1a
1. Statistical analysis revealed here that the formation of 61 times (R)-2 and 58 times (S)-2 under solid–vapor phase reaction of pyrimidine-5-carbaldehyde 1 and i-Pr2Zn exhibits statistical distribution and satisfies one of the necessary conditions of spontaneous absolute asymmetric synthesis as defined by Mislow.1a
The structure of a single crystal of carbaldehyde 1 belongs to achiral P21/n (Fig. S1, ESI†).10 Regarding the powder crystal structure of pyrimidine-5-carbaldehyde 1, we confirmed that the powder crystal of 1 is also achiral. The powder crystal of carbaldehyde 1 that was used in our present experiments was prepared by sublimation of crashed single crystals of 1. Using X-ray diffractometry, we compared the sublimed powder crystal of 1 (Fig. S2b, ESI†) with the powder prepared by crashing an achiral single crystal of 1 (Fig. S2a, ESI†). The diffraction pattern in Fig. S2b (ESI†) is consistent with the observed pattern for a single achiral crystal of 1 (Fig. S2a, ESI†), although some diffractions and multiples in Fig. S2b (ESI†) are stronger than those in Fig. S2a, ESI† because of the plate-like shape of the sublimed powder crystals.
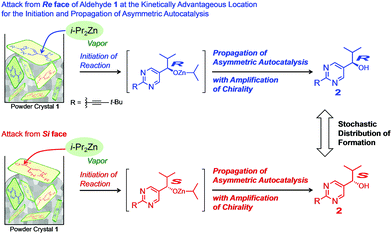
As shown in the figure of Table 1, under the present conditions, the surfaces of powder crystals of pyrimidine-5-carbaldehyde 1 are randomly oriented. Hence the exposure of enantiotopic Re and Si faces of pyrimidine-5-carbaldehyde 1 should also be random. Thus, it is conceivable that the kinetically first attack(s) of i-Pr2Zn vapor on one (or a small number) of the carbaldehyde 1 molecule(s) give(s) the first zinc alkoxide of pyrimidyl alkanol 2, the absolute configuration of which is controlled by the random orientation of aldehyde 1. The ee of the alkanol 2 produced on the crystal surface is amplified by the subsequent asymmetric autocatalysis. Simultaneously, the chirality of the generated alkanols 2 would be propagated in the glass tube. It should be noted that the ee of the formed pyrimidyl alkanol 2 can be easily amplified to very high ee (up to >99.5% ee) by consecutive asymmetric autocatalysis.4c
The initial kinetically controlled attack of i-Pr2Zn vapor on the randomly oriented powder crystal of pyrimidine-5-carbaldehyde 1 affords (R)- or (S)-isopropylzinc alkoxide of pyrimidyl alkanol 2, depending on the orientation of the Re or Si faces of aldehyde 1 for the exposure to the i-Pr2Zn vapor.11 Attack from the Si and Re faces of carbaldehyde 1 affords (S)- and (R)-pyrimidyl alkanol 1, respectively. The following asymmetric autocatalysis with amplification of chirality in the mixture of reagents and species4d affords enantioenriched pyrimidyl alkanol 2. Although each reaction is kinetically controlled, the distribution of total frequencies of the formation of (S)- or (R)-pyrimidyl alkanol 2 is stochastic.
In summary, we have demonstrated spontaneous absolute asymmetric synthesis in conjunction with asymmetric autocatalysis of pyrimidyl alkanol 2 from achiral powder-like crystals of pyrimidine-5-carbaldehyde 1 and i-Pr2Zn vapor. Without a chiral auxiliary, stochastic formation of (R)- and (S)-alkanol 2 was observed. Moreover, Pearson's chi-square test revealed that there was no deviation in the distribution of absolute configurations of alkanol 2. It is conceivable that the heterogeneous solid–vapor phase reaction is initiated under kinetic conditions. We postulate that chirality of the produced alkanol 2 is generated at almost the first attack of i-Pr2Zn to the Re or Si crystal surface of carbaldehyde 1, located in the most suitable position for the initiation, and the subsequent propagation of asymmetric autocatalysis with amplification of ee. Each reaction is controlled kinetically. In total, most results indicate the stochastic distribution of enantiomeric product.
To the best of our knowledge, our results provide the first example of the spontaneous absolute asymmetric synthesis achieved under heterogeneous solid–vapor phase conditions. Furthermore, the heterogeneous reaction under solid–vapor phase conditions could possibly also take place in more spacious platform12 than in a homogeneous reaction in a vessel of restricted size.
This work has been financially supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS KAKENHI Grant Numbers 23685012, 26810026 & 15H03781, Grant-in-Aid for JSPS Research Fellow to Y. K. Number 15J03968) and MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) K. Mislow, Collect. Czech. Chem. Commun., 2003, 68, 849 CrossRef CAS; (b) D. K. Kondepudi, R. J. Kaufman and N. Singh, Science, 1990, 250, 975 CrossRef CAS PubMed; (c) M. Bolli, R. Micura and A. Eschenmoser, Chem. Biol., 1997, 4, 309 CrossRef CAS; (d) J. S. Siegel, Chirality, 1998, 10, 24 CrossRef CAS; (e) B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418 CrossRef; (f) J. M. Ribó, J. Crusats, F. Sagués, J. Claret and R. Rubires, Science, 2001, 292, 2063 CrossRef; (g) H. Zepik, E. Shavit, M. Tang, T. R. Jensen, K. Kjaer, G. Bolbach, L. Leiserowitz, I. Weissbuch and M. Lahav, Science, 2002, 295, 1266 CrossRef CAS PubMed; (h) S. Pizzarello and A. L. Weber, Science, 2004, 303, 1151 CrossRef CAS PubMed; (i) C. Viedma, Phys. Rev. Lett., 2005, 94, 065504 CrossRef PubMed; (j) I. Weissbuch and M. Lahav, Chem. Rev., 2011, 111, 3236 CrossRef CAS; (k) K.-H. Ernst, Phys. Status Solidi B, 2012, 249, 2057 CrossRef CAS; (l) V. A. Soloshonok, C. Roussel, O. Kitagawa and A. E. Sorochinsky, Chem. Soc. Rev., 2012, 41, 4180 RSC; (m) Y. Saito and H. Hyuga, Rev. Mod. Phys., 2013, 85, 603 CrossRef CAS; (n) J. M. Ribó, C. Blanco, J. Crusats, Z. El-Hachemi, D. Hochberg and A. Moyano, Chem. – Eur. J., 2014, 20, 17250 CrossRef PubMed; (o) S. Olsson, P. M. Björemark, T. Kokoli, J. Sundberg, A. Lennartson, C. J. McKenzie and M. Håkansson, Chem. – Eur. J., 2015, 21, 5211 CrossRef CAS PubMed; (p) M. Quack, Angew. Chem., Int. Ed., 2002, 41, 4619 CrossRef PubMed; (q) K.-H. Ernst and A. J. Gellman, Catal. Lett., 2018, 148, 1610 CrossRef; (r) R. Raval, Chem. Soc. Rev., 2009, 38, 707 RSC; (s) A. Gonzalez-Campo and D. B. Amabilino, Top. Curr. Chem., 2013, 333, 109 CrossRef CAS PubMed.
- (a) T. Satyanarayana, S. Abraham and H. B. Kagan, Angew. Chem., Int. Ed., 2009, 48, 456 CrossRef CAS PubMed; (b) M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3138 CrossRef; (c) R. Breslow and Z.-L. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5723 CrossRef CAS PubMed; (d) Y. Hayashi, M. Matsuzawa, J. Yamaguchi, S. Yonehara, Y. Matsumoto, M. Shoji, D. Hashizume and H. Koshino, Angew. Chem., Int. Ed., 2006, 45, 4593 CrossRef CAS PubMed; (e) S. Cantekin, D. W. R. Balkenende, M. M. J. Smulders, A. R. A. Palmans and E. W. Meijer, Nat. Chem., 2011, 3, 42 CrossRef CAS PubMed; (f) M. Klussmann, H. Iwamura, S. P. Mathew, D. H. Wells Jr., U. Pandya, A. Armstrong and D. G. Blackmond, Nature, 2006, 441, 621 CrossRef CAS PubMed; (g) V. A. Soloshonok, H. Ueki, M. Yasumoto, S. Mekala, J. S. Hirschi and D. A. Singleton, J. Am. Chem. Soc., 2007, 129, 12112 CrossRef CAS PubMed; (h) E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752 CrossRef CAS PubMed; (i) C. Moberg, Acc. Chem. Res., 2016, 49, 2736 CrossRef CAS PubMed.
- For the recent reviews, see: (a) J. Han, A. Wzorek, V. A. Soloshonok and K. D. Klika, Electrophoresis, 2019 DOI:10.1002/elps.201800414; (b) J. Han, O. Kitagawa, A. Wzorek, K. D. Klika and V. A. Soloshonok, Chem. Sci., 2018, 9, 1718 RSC; (c) J. Han, V. A. Soloshonok, K. D. Klika, J. Drabowicz and A. Wzorek, Chem. Soc. Rev., 2018, 47, 1307 RSC.
- (a) K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767 CrossRef CAS; (b) T. Shibata, S. Yonekubo and K. Soai, Angew. Chem., Int. Ed., 1999, 38, 659 CrossRef CAS; (c) I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315 CrossRef CAS PubMed; (d) A. Matsumoto, T. Abe, A. Hara, T. Tobita, T. Sasagawa, T. Kawasaki and K. Soai, Angew. Chem., Int. Ed., 2015, 54, 15218 CrossRef CAS PubMed.
- (a) K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1 CrossRef CAS; (b) T. Kawasaki and K. Soai, Bull. Chem. Soc. Jpn., 2011, 84, 879 CrossRef CAS; (c) K. Soai and T. Kawasaki, in The Soai Reaction and Related Topic, ed. G. Pályi, C. Zicchi and L. Caglioti, Academia Nationale di Scienze Lettere e Arti (Modena), Modena, Edizioni Artestampa, 2012, pp. 9–34 Search PubMed; (d) T. Kawasaki and K. Soai, Isr. J. Chem., 2012, 52, 582 CrossRef CAS; (e) K. Soai and T. Kawasaki, Top. Organomet. Chem., 2013, 44, 261 CrossRef CAS; (f) K. Soai, T. Kawasaki and A. Matsumoto, Chem. Rec., 2014, 14, 70 CrossRef CAS PubMed; (g) K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643 CrossRef CAS PubMed; (h) K. Soai, A. Matsumoto and T. Kawasaki, in Advances in Asymmetric Autocatalysis and Related Topics, ed. G. Pályi, R. Kurdi and C. Zucchi, Academic Press, London, 2017, ch. 1, pp. 1–30 Search PubMed; (i) K. Soai, T. Kawasaki and A. Matsumoto, Tetrahedron, 2018, 74, 1973 CrossRef CAS; (j) K. Soai, Proc. Jpn. Acad., Ser. B, 2019, 95, 89 CrossRef PubMed.
- (a) M. Avalos, R. Babiano, P. Cintas, J. L. Jiménez and J. C. Palacios, Chem. Commun., 2000, 887 RSC; (b) D. G. Blackmond, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5732 CrossRef CAS PubMed; (c) J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776 CrossRef CAS PubMed; (d) J. M. Brown, I. Gridnev and J. Klankermayer, Top. Curr. Chem., 2008, 284, 35 CrossRef CAS; (e) T. Gehring, M. Busch, M. Schlageter and D. Weingand, Chirality, 2010, 22, E173 CrossRef CAS PubMed; (f) B. Barabás, J. Tóth and G. Pályi, J. Math. Chem., 2010, 48, 457 CrossRef; (g) E. Doka and G. Lente, J. Am. Chem. Soc., 2011, 133, 17878 CrossRef CAS PubMed; (h) J.-C. Micheau, C. Coudret and T. Buhse, in The Soai Reaction and Related Topic, ed. G. Pályi, C. Zucchi and L. Caglioti, Accad. Nazl. Sci. Lett. Arti, Editioni Artestampa, Modena, 2012, pp. 169–196 Search PubMed; (i) A. J. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800 CrossRef CAS PubMed; (j) O. Fülöp and B. Barabás, J. Math. Chem., 2016, 54, 10 CrossRef; (k) M. H. Todd, Chem. Soc. Rev., 2002, 31, 211 RSC; (l) M. Funes-Maldonado, B. Sieng and M. Amedjkouh, Org. Lett., 2016, 18, 2536 CrossRef CAS PubMed; (m) I. D. Gridnev and A. K. Vorobiev, ACS Catal., 2012, 2, 2137 CrossRef CAS; (n) T. Gehring, M. Quaranta, B. Odell, D. G. Blackmond and J. M. Brown, Angew. Chem., Int. Ed., 2012, 51, 9539 CrossRef CAS PubMed; (o) B. Barabás, R. Kurdi, C. Zucchi and G. Pályi, Chirality, 2018, 30, 913 CrossRef PubMed; (p) N. A. Hawbaker and D. G. Blackmond, ACS Cent. Sci., 2018, 4, 776 CrossRef CAS PubMed.
- (a) K. Soai, T. Shibata and Y. Kowata, Japan Kokai Tokkyo Koho, JP1997-268179, 1997 (priority date; 1996-02-01). An abstract is readily available as JPH09268179 from the European Patent Office http://worldwide.espacenet.com Search PubMed; (b) K. Soai, I. Sato, T. Shibata, S. Komiya, M. Hayashi, Y. Matsueda, H. Imamura, T. Hayase, H. Morioka, H. Tabira, J. Yamamoto and Y. Kowata, Tetrahedron: Asymmetry, 2003, 14, 185 CrossRef CAS; (c) D. A. Singleton and L. K. Vo, Org. Lett., 2003, 5, 4337 CrossRef CAS PubMed; (d) K. Suzuki, K. Hatase, D. Nishiyama, T. Kawasaki and K. Soai, J. Syst. Chem., 2010, 1, 5 CrossRef CAS.
- For the absolute asymmetric synthesis in the presence of amorphous silica gel, see: T. Kawasaki, K. Suzuki, M. Shimizu, K. Ishikawa and K. Soai, Chirality, 2006, 18, 479 CrossRef CAS PubMed.
- (a) W. H. Mills, Chem. Ind., 1932, 750–759 CrossRef CAS; (b) M. Maioli, G. Varadi, R. Kurdi, L. Caglioti and G. Pályi, J. Phys. Chem. B, 2016, 120, 7438 CrossRef CAS PubMed.
- CCDC 1524544†.
- For the attack from enantiotopic face of single crystal, see: T. Kawasaki, S. Kamimura, A. Amihara, K. Suzuki and K. Soai, Angew. Chem., Int. Ed., 2011, 50, 6796 CrossRef CAS PubMed . The reaction mechanism of asymmetric autocatalysis at the crystal interface is now under investigation. Our hypothesis: vapors of i-Pr2Zn and toluene make the crystal surface of aldehyde 1 somewhat wet, which provides the suitable surface environment for asymmetric autocatalysis.
- (a) I. Myrgorodska, C. Meinert, Z. Martins, L. L. S. d'Hendecourt and U. J. Meierhenrich, Angew. Chem., Int. Ed., 2015, 54, 1402 CrossRef CAS PubMed; (b) S. Pizzarello, Isr. J. Chem., 2016, 56, 1027 CrossRef CAS; (c) B. A. McGuire, P. B. Carroll, R. A. Loomis, I. A. Finneran, P. R. Jewell1, A. J. Remijan and G. A. Blake, Science, 2016, 352, 1449 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure of asymmetric autocatalysis, all experimental results and statistical analyses. CCDC 1524544. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc01875a |
| ‡ Present address: Department of Chemistry, Biology and Environmental Science, Nara Women's University, Kita-Uoya Nishi-machi, Nara, 630-8506, Japan. |
| This journal is © The Royal Society of Chemistry 2019 |