Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recognition and protection of glycosphingolipids by synthetic nanoparticle receptors†
Roshan W.
Gunasekara
and
Yan
Zhao
 *
*
Department of Chemistry, Iowa State University, Ames, Iowa 50011-3111, USA. E-mail: zhaoy@iastate.edu; Fax: +1-515-294-0105; Tel: +1-515-294-5845
First published on 28th March 2019
Abstract
Nanoparticle receptors were synthesized through micellar imprinting to bind glycosphingolipids with 20–140 μM binding affinities, meanwhile distinguishing glycan composition, the number of acyl chains, and hydroxylation of acyl chains in the lipids. The strong binding enabled the receptors to protect their target glycolipids dispersed in lipid membranes from enzymatic degradation.
Glycosphingolipids are abundant in the plasma membrane of vertebrate tissues. In addition to promoting membrane packing and raft formation, they play important roles in cell signaling and regulation.1 Their metabolites, including sphingosine and lysosphingolipids, also have a plethora of biological functions including the regulation of cell growth, survival, immune cell trafficking, and development of inflammation and cancer.2–4 Although synthetic receptors for these molecules could have many potential applications,5–9 selective recognition of glycosphingolipids (and carbohydrates in general) has been difficult because of the strong solvation of glycans in water and the subtle difference of their structures, often by the stereochemistry of a single hydroxyl.
In this work, we report cross-linked micelles for the selective binding of several important glycosphingolipids (1–5). Among them, kerasin (1) and phrenosin (2) are major lipids in the brain. Their misregulation is related to Krabbe disease and their metabolite psychosine (3) is highly cytotoxic.10,11 Gaucher's disease causes abnormal accumulation of glucosylceramide 4 in the liver and spleen.12 Lactosylceramide (5) is a biological precursor to more complex glycolipids and is also involved in multiple signal transduction pathways.13 As natural lipids, the acyl groups of 1, 2, 4, and 5 consist of mixtures of fatty acid chains with mostly 16–24 carbons and 0 or 1 degree of unsaturation. Psychosine has the acyl group removed.
Scheme 1 shows the preparation of molecularly imprinted nanoparticle (MINP) receptors for these lipids. A glycosphingolipid, after hydrogenation (to prevent unsaturated lipid tails from participating in free radical polymerization), first forms a complex with functional monomer (FM) 8. The boroxole14,15 is known to form boronate esters with sugar in situ under micellar conditions,16 similar to other boronic acid-derived sugar-binding molecules.17–19 The complex is incorporated into the mixed micelle of 6 and 7, along with DVB and DMPA (a photoinitiator). The micelle, with many alkynes and azides on the surface, is cross-linked on the surface by the Cu(I)-catalyzed click reaction and then in the core by photo-induced free radical polymerization. In the final step, monoazide 9 is “clicked” onto the cross-linked micelle to enhance its hydrophilicity.20
The characterization of MINPs is reported in the ESI† (Fig. S2–S17). Their sizes (4–5 nm) were determined by DLS and had been confirmed by TEM.21,22 Their bindings with 1–5 were determined by isothermal titration calorimetry (ITC) and are summarized in Table 1. Because the guest molecules were insoluble in water, we added 0.1% Tween 20 to the HEPES buffer in the ITC titrations—a common way to solubilize lipids.23
| Entry | MINP | Guest | K a (× 103 M−1) | CRR | −ΔG (kcal mol−1) | ΔΔG (kcal mol−1) | −ΔH (kcal mol−1) | TΔS (kcal mol−1) | N |
|---|---|---|---|---|---|---|---|---|---|
a All MINPs were prepared with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 FM/template ratio. A 50 1 FM/template ratio. A 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 surfactant/template ratio was used to give an average of one binding site per MINP, as the nanoparticle contains ∼50 cross-linked surfactants. The binding constants were determined by ITC in 10 mM HEPES buffer (pH 7.4) containing 0.1% Tween 20 detergent. CRR is the cross-reactivity ratio, defined as the binding constant of a guest relative to that of the template for a particular MINP. ΔΔG = ΔG(guest) − ΔG(template). N is the number of binding sites per MINP.
b The binding stoichiometry could not be determined accurately because the binding was too weak. 1 surfactant/template ratio was used to give an average of one binding site per MINP, as the nanoparticle contains ∼50 cross-linked surfactants. The binding constants were determined by ITC in 10 mM HEPES buffer (pH 7.4) containing 0.1% Tween 20 detergent. CRR is the cross-reactivity ratio, defined as the binding constant of a guest relative to that of the template for a particular MINP. ΔΔG = ΔG(guest) − ΔG(template). N is the number of binding sites per MINP.
b The binding stoichiometry could not be determined accurately because the binding was too weak.
|
|||||||||
| 1 | MINP(1) | 1 | 52.8 ± 5.8 | 1.00 | 6.44 | 0.00 | 3.35 ± 0.27 | 3.09 | 0.9 ± 0.1 |
| 2 | MINP(1) | 2 | 26.4 ± 1.8 | 0.50 | 6.03 | 0.41 | 1.78 ± 0.27 | 4.25 | 1.0 ± 01 |
| 3 | MINP(1) | 3 | 6.01 ± 0.19 | 0.11 | 5.15 | 1.29 | 1.44 ± 0.12 | 3.71 | 1.2 ± 0.1 |
| 4 | MINP(1) | 4 | 0.045 ± 0.001 | 0.00 | 2.25 | 4.18 | 0.089 ± 0.002 | 2.16 | —b |
| 5 | MINP(1) | 5 | 0.006 ± 0.002 | 0.00 | 1.10 | 5.34 | 0.085 ± 0.005 | 1.01 | —b |
| 6 | MINP(2) | 1 | 9.65 ± 0.10 | 0.33 | 5.43 | 0.65 | 1.02 ± 0.14 | 4.41 | 1.0 ± 0.1 |
| 7 | MINP(2) | 2 | 28.9 ± 2.3 | 1.00 | 6.08 | 0.00 | 2.01 ± 0.78 | 4.07 | 0.9 ± 0.1 |
| 8 | MINP(2) | 3 | 5.26 ± 0.19 | 0.18 | 5.07 | 1.01 | 1.48 ± 0.15 | 3.59 | 1.0 ± 0.1 |
| 9 | MINP(2) | 4 | 0.015 ± 0.002 | 0.00 | 1.61 | 4.47 | 0.029 ± 0.003 | 1.58 | —b |
| 10 | MINP(2) | 5 | 0.006 ± 0.001 | 0.00 | 1.09 | 4.99 | 0.021 ± 0.004 | 1.07 | —b |
| 11 | MINP(3) | 1 | 2.13 ± 0.28 | 0.24 | 4.54 | 0.84 | 0.55 ± 0.02 | 3.99 | 1.1 ± 0.2 |
| 12 | MINP(3) | 2 | 1.42 ± 0.05 | 0.16 | 4.30 | 1.08 | 0.54 ± 0.02 | 3.76 | 0.8 ± 0.1 |
| 13 | MINP(3) | 3 | 8.85 ± 0.13 | 1.00 | 5.38 | 0.00 | 1.63 ± 0.15 | 3.74 | 1.1 ± 0.1 |
| 14 | MINP(3) | 4 | 0.024 ± 0.001 | 0.00 | 1.89 | 3.49 | 0.10 ± 0.01 | 1.79 | —b |
| 15 | MINP(3) | 5 | 0.008 ± 0.001 | 0.00 | 1.24 | 4.14 | 0.019 ± 0.001 | 1.22 | —b |
| 16 | MINP(4) | 1 | 0.011 ± 0.002 | 0.00 | 1.44 | 3.79 | 0.075 ± 0.004 | 1.37 | —b |
| 17 | MINP(4) | 2 | 0.015 ± 0.002 | 0.00 | 1.62 | 3.62 | 0.033 ± 0.001 | 1.59 | —b |
| 18 | MINP(4) | 3 | 0.026 ± 0.005 | 0.00 | 1.93 | 3.31 | 0.069 ± 0.003 | 1.86 | —b |
| 19 | MINP(4) | 4 | 6.94 ± 0.11 | 1.00 | 5.24 | 0.00 | 1.50 ± 0.03 | 3.73 | 0.9 ± 0.1 |
| 20 | MINP(4) | 5 | 0.027 ± 0.004 | 0.00 | 1.96 | 3.28 | 0.090 ± 0.005 | 1.87 | —b |
| 21 | MINP(5) | 1 | 2.35 ± 0.07 | 0.25 | 4.60 | 0.82 | 0.39 ± 0.02 | 4.21 | 1.1 ± 0.1 |
| 22 | MINP(5) | 2 | 1.41 ± 0.05 | 0.15 | 4.29 | 1.12 | 1.01 ± 0.01 | 3.28 | 0.9 ± 0.1 |
| 23 | MINP(5) | 3 | 0.004 ± 0.002 | 0.00 | 0.84 | 4.58 | 0.072 ± 0.01 | 0.77 | —b |
| 24 | MINP(5) | 4 | 0.009 ± 0.002 | 0.00 | 1.31 | 4.10 | 0.024 ± 0.008 | 1.29 | 0.4 ± 0.2 |
| 25 | MINP(5) | 5 | 9.42 ± 0.21 | 1.00 | 5.42 | 0.00 | 1.10 ± 0.06 | 4.32 | 0.9 ± 0.2 |
MINP(1), i.e., MINP prepared with kerasin 1 as the template, bound the template with Ka = 52.8 × 103 M−1 (entry 1). The binding constant is very competitive with those by natural lectin for a monosaccharide (Ka = 103–104 M−1).5,6 Consistent with successful imprinting, the CRR (cross-reactivity ratio) decreased significantly for other lipids. The very similar phrenosin 2, having a single extra hydroxyl group on the acyl chain, gave a CRR of 0.50 (entry 2). Removal of the acyl chain was even less tolerated, affording a CRR of 0.11 for 3 (entry 3).
In aqueous solution, hydrophobic interactions dominate in the imprinting and binding of MINPs.24 When (hydrogenated) 1 was used as the template, everything including the hydrophobic tails should be imprinted. A change from 1 to 3, which misses a long acyl chain, however, only changed the binding energy of MINP(1) by 1.29 kcal mol−1 (entry 3). The small ΔΔG was inconsistent with the hydrophobic tail being involved in the binding. The conclusion is also supported by the overall binding energy of 1 by MINP(1). Because transfer of hexane to water costs 0.92 kcal mol−1 per methylene,25 burying two long hydrocarbon tails would have yielded a much larger binding energy than the observed value of 6.44 kcal mol−1. In our experiments, the hydrophobic tails of the lipid were solubilized by the hydrophobic core of the Tween micelle prior to binding. Apparently, once their hydrophobic needs were satisfied, there was no driving force to make the tails come out of the micelle and go into the imprinted sites (vide infra for additional discussion).
Gratifyingly, MINP(1) was extremely sensitive to the glycan, as 4 and 5 showed practically no binding. The selectivity in binding was >1000 for the glucosylceramide and lactosylceramide (entries 4 and 5). The selectivity was significant because 4 only differs from 1 in the stereochemistry of a single hydroxyl (on C4 of the glycoside). The large difference in binding suggests that this particular hydroxyl was involved in boronate formation.16,26 It is also significant that 5 was not bound at all. Even though the lactosylceramide contains a β-galactoside and thus has the correct diols to bind the boroxole, its large glycan precluded it from entering the small imprinted site created for 1.
MINP(2) displayed similar trends in binding and selectivity to MINP(1) (entries 6–10). For MINP(3), since its template does not have an acyl chain, it would not be able to bind 1 and 2 if it had to accommodate their long acyl group. Entries 11–12 show that MINP(3) exhibited a weaker but clearly observable binding for these acyl-containing lipids, thus supporting the earlier conclusion that the hydrophobic tails of the lipids stayed in the Tween micelle during binding and the binding was mainly derived from the glycan and its neighboring groups.
MINP(4) bound no other sphingolipids except its own template. The results once again highlighted the selectivity of MINPs for glycan, as 1 only differed from 4 in the stereochemistry of one hydroxyl.
Lactosylceramide 5 on its galactoside has the same boroxole-binding cis-3,4-diol16 as 1. MINP(5), thus, has the appropriate boroxole in its binding pocket to bind the glycan of 1 even though the pocket was created for the disaccharide. Indeed, weaker but significant binding was obtained with this MINP for galactosylceramides 1 and 2 (entries 21 and 22). The fact that 1 and 2 showed appreciable binding suggests that boronate formation was key to the binding. It was initially puzzling to us that the galactosyl-containing psychosine 3 was bound extremely weakly (entry 23). However, given that the compound had both a shortened glycan and a missing acyl chain in comparison to 5, the difference might be just too large for MINP(5) to tolerate.
For all the bindings in Table 1, the entropic term (TΔS) was positive (favorable). The result was reasonable because a large number of water molecules would be released to bulk upon binding. Interestingly, the binding enthalpy (−ΔH) was always the highest for the template over other guests, suggesting that correct boronate bond formation was important to the binding.
Tween is nonionic but MINP is anionic due to the excess of anionic surfactant 6 used in the preparation (Fig. S2, zeta-potential = −34.6 mV, ESI†). Because many biological membranes are negatively charged, we could use their electric potential to modulate the binding of MINPs for a sphingolipid. This strategy could be very useful in biological applications, as alkynyl-functionalized cross-linked micelles can be functionalized with different surface groups to modulate their interactions with lipid membranes.27
Table 2 compares the binding of MINP(4) for 4 when solubilized by Tween and liposomes made from POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) and POPG (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol). POPC is a zwitterionic lipid and POPG is anionic. As shown by entries 2–5, an increase of POPG in the membrane steadily decreased the binding, consistent with an electrostatic model.
| Entry | MINP | Guest | Solution | K a (× 103 M−1) | CRR | −ΔG (kcal mol−1) | −ΔH (kcal mol−1) | TΔS (kcal mol−1) | N |
|---|---|---|---|---|---|---|---|---|---|
| a All binding studies were determined by ITC in 10 mM HEPES buffer (pH 7.4) containing 0.1% Tween-20 detergent. CRR is the cross-reactivity ratio, defined as the binding constant of a guest relative to that of the template for a particular MINP. N is the number of binding sites per MINP. b The binding stoichiometry could not be determined accurately because the binding was too weak. | |||||||||
| 1 | MINP(4) | 4 | 0.1% Tween-20 | 6.94 ± 0.11 | 1.00 | 5.24 | 1.50 ± 0.03 | 3.73 | 0.9 ± 0.1 |
| 2 | MINP(4) | 4 | 1% POPC | 5.98 ± 0.72 | 0.86 | 5.15 | 0.80 ± 0.07 | 4.35 | 1.1 ± 0.1 |
| 3 | MINP(4) | 4 | 1% 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPC/POPG 1 POPC/POPG |
5.44 ± 0.56 | 0.78 | 5.09 | 1.31 ± 0.06 | 3.78 | 1.0 ± 0.1 |
| 4 | MINP(4) | 4 | 1% 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPC/POPG 1 POPC/POPG |
1.77 ± 0.06 | 0.26 | 4.43 | 0.72 ± 0.01 | 3.71 | 1.0 ± 0.1 |
| 5 | MINP(4) | 4 | 1% POPG | 0.045 ± 0.008 | 0.01 | 2.25 | 0.18 ± 0.01 | 2.07 | —b |
| 6 | MINP(10) | 10 | 1% POPC | 3.58 ± 0.35 | 1.00 | 4.84 | 1.39 ± 0.15 | 3.46 | 1.2 ± 0.1 |
| 7 | MINP(10) | 4 | 1% POPC | 1.71 ± 0.08 | 0.48 | 4.41 | 0.91 ± 0.06 | 3.49 | 1.0 ± 0.1 |
| 8 | MINP(10) | 10 | HEPES buffer | 19.5 ± 1.7 | 5.45 | 5.85 | 1.60 ± 0.11 | 4.24 | 1.1 ± 0.1 |
| 9 | MINP(11) | 11 | HEPES buffer | 30.3 ± 1.1 | 1.00 | 6.11 | 1.81 ± 0.06 | 4.30 | 0.9 ± 0.1 |
| 10 | MINP(11) | 10 | HEPES buffer | 10.5 ± 1.0 | 0.35 | 5.48 | 4.30 ± 0.21 | 1.18 | 1.0 ± 0.1 |
| 11 | MINP(11) | 10 | 1% POPC | 2.01 ± 0.06 | 0.07 | 4.50 | 0.86 ± 0.03 | 3.64 | 1.0 ± 0.1 |
| 12 | MINP(11) | 4 | 1% POPC | 2.34 ± 0.20 | 0.08 | 4.59 | 0.61 ± 0.05 | 3.98 | 1.0 ± 0.1 |
Because both sphingolipids and their metabolites are important signaling and regulatory molecules,2–4 it is very useful to have chemical tools to control their enzymatic processing. Galactosyl lipids may be detected by Amplex Red assay,28 which uses a galactose oxidase to oxidize the sugar into the aldehyde derivative. The hydrogen peroxide byproduct generated meanwhile is used by horseradish peroxidase to oxidize Amplex Red into a fluorescent product, resorufin.
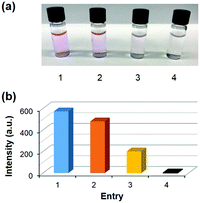
Fig. 1a shows four samples of Amplex Red assay after 35 min reaction time. As the photographs show, the enzymes produced more resorufin product (pink in color) with 1 solubilized in Tween 20 than in POPC. We attributed the difference to the different accessibility of the glycan in the micelle and in lipid membranes to galactose oxidase. Addition of 280 μM MINP(1) made the color much fainter, indicating slower oxidation of the galactoside. The last sample was a blank, showing no product formation in the absence of 1. Fig. 1b shows the emission intensities of resorufin in the four samples. Based on the intensities, 280 μM MINP(1) slowed down the oxidation of the galactosylceramide by more than 50%.
We also recorded the fluorescence spectra of a series of kerasin/Amplex Red samples with the concentration of MINP(1) varied from 0 to 280 μM (Fig. S45a, ESI†). As the concentration of the nanoparticle receptor increased, oxidation of 1 in the POPC lipid membranes decreased consistently. When we plotted the emission intensity at 585 nm at 35 min reaction time against the concentration of MINP, the intensities could be fitted nearly perfectly to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm (Fig. S45b, ESI†). The apparent “binding constant” obtained was Ka = (5.42 ± 0.75) × 103 M−1. Even though this number was lower than the actual binding constant (52.8 × 103 M−1) determined by ITC (Table 1, entry 1), the correlation does indicate that protection of the glycolipid from enzymatic degradation was a direct result of binding.
1 binding isotherm (Fig. S45b, ESI†). The apparent “binding constant” obtained was Ka = (5.42 ± 0.75) × 103 M−1. Even though this number was lower than the actual binding constant (52.8 × 103 M−1) determined by ITC (Table 1, entry 1), the correlation does indicate that protection of the glycolipid from enzymatic degradation was a direct result of binding.
Natural lipids are often very expensive and sometimes difficult to obtain. Since our binding data suggested that MINPs recognized the sphingolipids mainly around their glycans, we decided to employ simple glycosides such as 10 and 11 to prepare MINPs for recognizing glucosylceramide 4.
Table 2 shows that MINP(10) bound 4 indeed with a substantial binding constant (Ka = 1710 M−1, entry 7). Although the Ka value was half of that for the template itself (entry 6), and also weaker than that for 4 by MINP(4) under similar conditions (Ka = 5980 M−1, entry 2), the submillimolar affinity was impressive given that an analogue was used instead of the template. Because 10 was soluble in water, we also determined its binding by MINP (10) in HEPES buffer, without any additives (Tween or POPC). The binding constant was 5.45 times higher than that with POPC in the solution (entry 8). Thus, once the hydrophobic tail (i.e., octyl) was exposed to water prior to binding, it contributed strongly to the binding, unlike the situation when the glycolipids were solubilized by Tween or POPC.
Compound 11 has a different aglycon from 10. MINP(11), expectedly, bound its template strongly in buffer (Ka = 30.3 × 103 M−1, entry 9). The imprinted pocket for the p-nitrophenyl group apparently was large enough for an octyl group to fold and fit in,29 as it bound 10 with 1/3 of the binding constant (entry 10). Once the binding was measured in the presence of POPC, we lost the contribution from the hydrophobic aglycon again and obtained a lower Ka (entry 11). Importantly, glucosylceramide 4 was still bound with Ka > 2000 M−1 (entry 12). Thus, to prepare imprinted receptors for natural sphingolipids, we only need to focus on the key glycan instead of all the structure—a feature extremely useful in practical applications.
We thank NIGMS (R01GM113883) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Chalfant and M. Del Poeta, Sphingolipids as signaling and regulatory molecules, Springer Science, New York, N.Y., 2010 Search PubMed.
- Y. Hannun and R. Bell, Science, 1989, 243, 500–507 CrossRef CAS.
- M. Maceyka and S. Spiegel, Nature, 2014, 510, 58 CrossRef CAS PubMed.
- T. Wennekes, R. J. B. H. N. van den Berg, R. G. Boot, G. A. van der Marel, H. S. Overkleeft and J. M. F. G. Aerts, Angew. Chem., Int. Ed., 2009, 48, 8848–8869 CrossRef CAS.
- J. P. Kamerling and G.-J. Boons, Comprehensive glycoscience: from chemistry to systems biology, Elsevier, Amsterdam, Boston, 1st edn, 2007 Search PubMed.
- B. Wang and G.-J. Boons, Carbohydrate recognition: biological problems, methods, and applications, Wiley, Hoboken, N.J., 2011 Search PubMed.
- A. P. Davis and T. D. James, Carbohydrate Receptors, Wiley-VCH, Weinheim, 2005 Search PubMed.
- S. Jin, Y. Cheng, S. Reid, M. Li and B. Wang, Med. Res. Rev., 2010, 30, 171–257 CAS.
- T. D. James, M. D. Phillips and S. Shinkai, Boronic acids in saccharide recognition, RSC Publishing, Cambridge, 2006 Search PubMed.
- L. Svennerholm, M. T. Vanier and J. E. Mansson, J. Lipid Res., 1980, 21, 53–64 CAS.
- A. B. White, F. Galbiati, M. I. Givogri, A. Lopez Rosas, X. Qiu, R. van Breemen and E. R. Bongarzone, J. Neurosci. Res., 2011, 89, 352–364 CrossRef CAS PubMed.
- A. Dandana, S. Ben Khelifa, H. Chahed, A. Miled and S. Ferchichi, Pathobiology, 2016, 83, 13–23 CrossRef PubMed.
- H. Nakamura, Y. Moriyama, T. Makiyama, S. Emori, H. Yamashita, R. Yamazaki and T. Murayama, J. Biol. Chem., 2013, 288, 23264–23272 CrossRef CAS PubMed.
- M. Dowlut and D. G. Hall, J. Am. Chem. Soc., 2006, 128, 4226–4227 CrossRef CAS PubMed.
- M. Bérubé, M. Dowlut and D. G. Hall, J. Org. Chem., 2008, 73, 6471–6479 CrossRef PubMed.
- R. W. Gunasekara and Y. Zhao, J. Am. Chem. Soc., 2017, 139, 829–835 CrossRef CAS PubMed.
- G. Wulff and W. Vesper, J. Chromatogr., 1978, 167, 171–186 CrossRef CAS.
- X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC.
- S. D. Bull, M. G. Davidson, J. M. H. Van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y. B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Z. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed.
- Y. Zhao, Langmuir, 2016, 32, 5703–5713 CrossRef CAS PubMed.
- S. Fa and Y. Zhao, Chem. Mater., 2017, 29, 9284–9291 CrossRef CAS PubMed.
- S. Fa and Y. Zhao, Chem. – Eur. J., 2018, 24, 150–158 CrossRef CAS PubMed.
- S. Schuck, M. Honsho, K. Ekroos, A. Shevchenko and K. Simons, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5795–5800 CrossRef CAS PubMed.
- J. K. Awino and Y. Zhao, J. Am. Chem. Soc., 2013, 135, 12552–12555 CrossRef CAS PubMed.
- M. H. Abraham, J. Am. Chem. Soc., 1982, 104, 2085–2094 CrossRef CAS.
- J. K. Awino, R. W. Gunasekara and Y. Zhao, J. Am. Chem. Soc., 2016, 138, 9759–9762 CrossRef CAS PubMed.
- X. Li and Y. Zhao, Bioconjugate Chem., 2012, 23, 1721–1725 CrossRef CAS PubMed.
- M. Fortelius and P. Mattjus, Chem. Phys. Lipids, 2006, 142, 103–110 CrossRef CAS PubMed.
- J. K. Awino and Y. Zhao, Org. Biomol. Chem., 2017, 15, 4851–4858 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, fluorescence titration curves, and additional figures. See DOI: 10.1039/c9cc01694e |
| This journal is © The Royal Society of Chemistry 2019 |