Open Access Article
Open Access ArticleSynthesis of germanium nanocrystals from solid-state disproportionation of a chloride-derived germania glass†
Yujie
Wang
 a,
Utkarsh
Ramesh
a,
Charles K. A.
Nyamekye
bc,
Bradley J.
Ryan
a,
Rainie D.
Nelson
a,
Utkarsh
Ramesh
a,
Charles K. A.
Nyamekye
bc,
Bradley J.
Ryan
a,
Rainie D.
Nelson
 a,
Abdulla M.
Alebri
a,
Umar H.
Hamdeh
a,
Atefe
Hadi
a,
Emily A.
Smith
a,
Abdulla M.
Alebri
a,
Umar H.
Hamdeh
a,
Atefe
Hadi
a,
Emily A.
Smith
 bc and
Matthew G.
Panthani
bc and
Matthew G.
Panthani
 *a
*a
aDepartment of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA. E-mail: panthani@iastate.edu
bDepartment of Chemistry, Iowa State University, Ames, IA 50011, USA
cAmes Laboratory, U.S. Department of Energy, Ames, IA 50011, USA
First published on 29th April 2019
Abstract
Germanium nanocrystals (Ge NCs) have potential to be used in several optoelectronic applications such as photodetectors and light-emitting diodes. Here, we report a solid-state route to synthesizing Ge NCs through thermal disproportionation of a germania (GeOX) glass, which was synthesized by hydrolyzing a GeCl2·dioxane complex. The GeOX glass synthesized in this manner was found to have residual Cl content. The process of nanocrystal nucleation and growth was monitored using powder X-ray diffraction, transmission electron microscopy, X-ray photoelectron spectroscopy and Raman spectroscopy. Compared to existing solid-state routes for synthesizing colloidal Ge NCs, this approach requires fewer steps and is amenable to scaling to large-scale reactions.
Quantum dots have tremendous potential for several optoelectronic applications due to their unique properties;1–3 however, many quantum dot materials contain toxic elements such as Cd and Pb. This has motivated a search for other nanocrystal systems, including Group IV semiconductors (Si, Ge, and Sn).4–6 Ge NCs are thought to be promising for a variety of applications such as light-emitting diodes,7,8 nonvolatile memory,9 field-effect transistors,10 lithium ion batteries,11 and near-infrared photodetectors.12
Several synthetic methods for preparing Ge NCs have been reported, including solution-phase synthesis from Zintl salts,13,14 plasma-based synthesis,15 thermal processing of sol–gel derived from organogermanium,16–18 decomposition of organogermanes in supercritical fluids,19 microwave-assisted colloidal synthesis,20,21 and hot-injection based arrested precipitation.7,22 Very recently, there have been reports of solid state approaches using a Ge(OH)2 precursor.23–25 These approaches used Ge(OH)2 that was derived from GeO2, followed by thermal annealing to induce dehydration and disproportionation, ultimately resulting in Ge NCs suspended within a germania matrix.
In this report, we departed from this strategy by synthesizing GeOX through the hydrolysis and subsequent dehydration of GeCl2.26 Compared to previous approaches, the GeCl2 precursor has the advantage of being hydrolyzed by water at room temperature to instantly form Ge(OH)2, which can subsequently be dehydrated to form GeOX. The GeOX was subsequently used as a solid-state precursor for synthesizing Ge NCs by thermal disproportionation. Freestanding Ge NCs could be liberated by mixing the oxide-embedded Ge NCs with deionized water to selectively etch the matrix. Further surface modification could be achieved by first hydrogen terminating the Ge NC surfaces using a hydrofluoric acid (HF) solution followed by heating in the presence of a terminal alkene to induce hydrogermylation, which resulted in alkyl-terminated Ge NCs. In a typical reaction, GeCl2·dioxane was hydrolyzed with water and dried under vacuum and heated to form a yellow powder that was subsequently annealed in N2 at elevated temperatures.
We use powder X-ray diffraction (XRD) to identify the processing temperature at which crystalline Ge begins to appear (Fig. 1). The XRD pattern of the hydrolyzed GeCl2·dioxane (prior to annealing) exhibits broad amorphous features centered around 31.4° and 50.8° 2θ. Previous studies from Javadi et al.24,25 and Sun et al.23 also reported amorphous features from GeO2-derived GeO prior to annealing; however these amorphous scattering features were at slightly lower angles (27.5° and 50° 2θ) and were attributed to amorphous germanium.24,25 The different positions of the amorphous features in the precursors indicate that our samples may possess a different composition from the previous studies. Differential scanning calorimetry (DSC) reveals an exotherm that we hypothesized to coincide with the decomposition of the precursor and formation of Ge nuclei around 250 °C (Fig. S1, ESI†). From XRD pattern, the precursor remains amorphous through dehydration at 250 °C, and upon continued heating up to 325 °C. Upon heating to 325 °C, peaks corresponding to diamond cubic Ge and hexagonal GeO2 emerge. At higher temperatures, the relative intensities of cubic Ge reflections increase relative to the features corresponding to the amorphous GeOX. There is relatively little signal indicating the presence of amorphous GeOX for samples processed at temperatures greater than 400 °C, suggesting that the GeOX is mostly converted to crystalline Ge and GeO2. We note the products that are exposed to environments with relatively high humidity (RH ≥ 50%) have reflections corresponding to hexagonal GeO2. However, samples that are prepared in low relative humidity (RH ≤ 20%) do not show any reflections corresponding to crystalline GeO2 (Fig. S2, ESI†), and rather only have broad amorphous features which we assign to amorphous GeOX, since these features have equivalent width and position to the hydrolyzed GeCl2·dioxane. These data suggest that the formation of crystalline GeO2 results from exposure to atmospheric moisture. This is in line with a previous experimental study demonstrating that water can cause the transformation of amorphous GeO2 to hexagonal crystalline GeO2.27
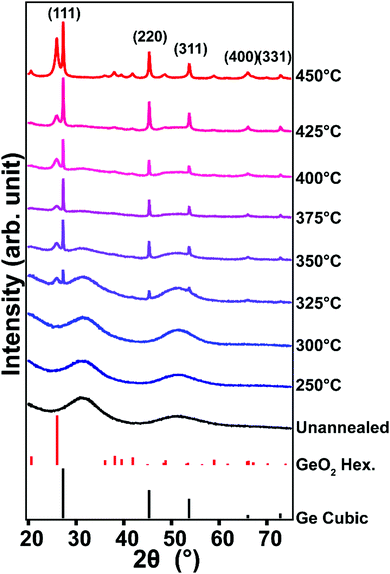 | ||
| Fig. 1 XRD patterns of hydrolyzed GeCl2·dioxane before and after annealing at various temperatures. Cubic Ge reference: PDF # 00-004-0545, hexagonal GeO2 reference: PDF # 00-036-1463. | ||
Transmission electron microscopy (TEM) confirms the presence of Ge NCs at annealing temperatures at or above 325 °C (Fig. 2 and Fig. S3, ESI†), with a measured d-spacing of 3.3 Å, consistent with the crystalline cubic Ge(111) plane. Particle size analysis of TEM images suggests that there is no trend in size with respect to processing temperature. This is different from the observation of Javadi et al. of a clear increase in nanocrystal size with increasing annealing temperature.25 The Ge NCs are polydisperse and have different morphologies, which could be caused by the two-step heating procedure.5 The presence of unreacted precursors and amorphous species are also observed in the vicinity of Ge NCs, the presence of which decreases with increasing reaction temperature (Fig. S4–S9, ESI†). This is consistent with XRD data that indicates the decrease of amorphous GeOX with increasing processing temperature (Fig. 1).
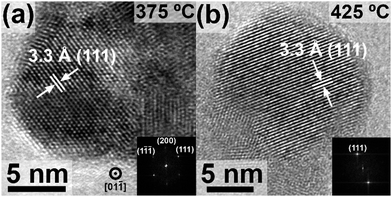 | ||
| Fig. 2 TEM images of nanocrystals obtained from hydrolyzed GeCl2·dioxane annealed at (a) 375 °C and (b) 425 °C. The insets show the corresponding FFT pattern indexed to diamond cubic Ge. | ||
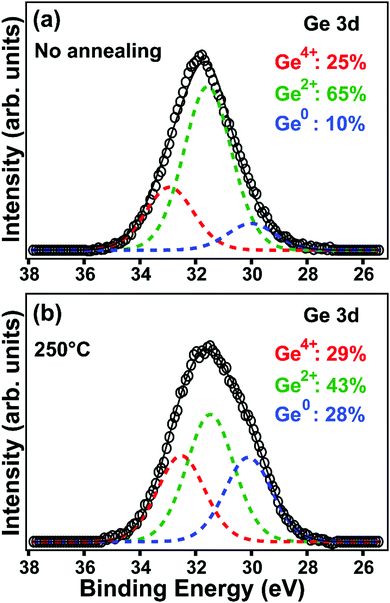
X-ray photoelectron spectroscopy (XPS) measurements quantify the distribution of oxidation states of hydrolyzed GeCl2·dioxane before and after annealing at 250 °C. The measurements suggest a disproportionation process occurs during thermal annealing (Fig. 3 and Fig. S12, ESI†). As shown in Fig. S12 (ESI†), prior to annealing, the hydrolyzed GeCl2·dioxane has signals from Ge, C, O as well as Cl. The presence of Cl likely indicates incomplete conversion of GeCl2·dioxane to Ge(OH)2 during the hydrolysis step. High-resolution XPS (Fig. 3) of the hydrolyzed GeCl2·dioxane before annealing has Ge 3d peak that is dominated by Ge2+, with smaller contributions from Ge4+ and Ge0.28 The Ge2+ signal is attributed to Ge(OH)2 from hydrolysis and unreacted GeCl2, while Ge4+ is attributed to surface oxidation. Due to the lack of crystalline features in XRD, the Ge0 signal likely arises from amorphous Ge from the precursor. After annealing at 250 °C, the peak becomes broader and signals from Ge0 and Ge4+ increases while signal from Ge2+ decreases, suggesting that the disproportionation process begins at temperatures as low as 250 °C, supporting the hypothesis that the exotherm in DSC 250 °C is related to the decomposition of the precursor and formation of Ge nuclei.
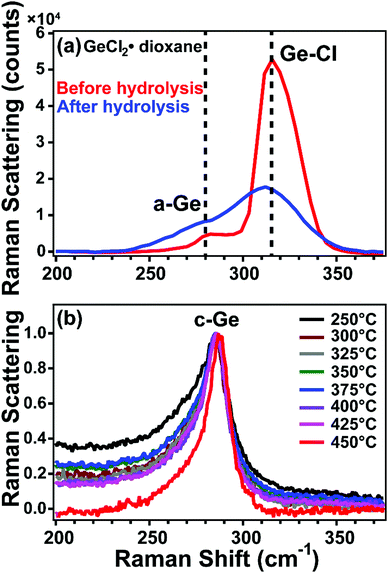
To better understand the formation of crystalline Ge at unprecedentedly low temperatures, we measured the Raman spectra of the GeCl2·dioxane precursor before and after hydrolysis (Fig. 4a). The peak of GeCl2·dioxane at 316 cm−1 is attributed to the Ge phonon mode of a Ge–Cl stretch,28 and the shoulder around 280 cm−1 is attributed to amorphous Ge.29,30 The entire Raman spectrum of GeCl2·dioxane from 200 to 4000 cm−1 is shown in Fig. S13 (ESI†) and detailed Raman peak assignments are shown in Table S1 (ESI†). After hydrolyzing the GeCl2·dioxane, the Ge–Cl stretch becomes relatively weak compared to the amorphous Ge signal, but is still present (Fig. 4a). This is consistent with the Cl signal seen in XPS and confirms the presence of GeCl2 before annealing (Fig. S12, ESI†). Furthermore, a new Raman peak associated with an O–H bond at 3510 cm−1 appears after hydrolysis (as shown in Fig. S14, ESI†), indicating that some of the GeCl2 reacted with water to form Ge(OH)2. The presence of the peak associated with amorphous Ge is consistent with the XPS spectra that has photoemission from Ge0 in the hydrolyzed GeCl2·dioxane (Fig. 3).
After thermally annealing the hydrolyzed precursor at temperatures greater than or equal to 250 °C, peaks corresponding to Ge–Ge begin to appear, with Raman shift of around 285 cm−1 (Fig. 4b). This presumably indicates the formation of small Ge NCs or possibly nuclei at temperatures as low as 250 °C, consistent with observation of exotherm in DSC and the Ge0 from XPS at 250 °C. As shown in Fig. 4b, the Raman peaks become more symmetric, sharper, and shift to higher wavenumbers with increasing processing temperatures (see Fig. S15 for more details, ESI†). Qualitatively, the change in peak shape indicates that the size of the Ge NCs increases with temperature, as is predicted by the phonon confinement model.30–32
Compared to previous reports of Ge NC synthesis from oxide disproportionation, the approach reported here results in the formation of crystalline Ge at relatively low temperatures.18,23,24,33 While further work must be done to compare the crystallization dynamics between these approaches, there are some hints as to what could cause the different crystallization temperature. As previously mentioned, the XRD pattern of the hydrolyzed product has different amorphous feature locations from previous reports.18,23,24,33 Also, Raman and XPS results imply the presence of Ge–Cl bonds (see Fig. S12 (ESI†) for the survey XP spectrum of hydrolyzed GeCl2·dioxane). Ge–Cl bonds have a lower dissociation energy than Ge–O (390.8 ± 9.6 kJ mol−1 and 652.7 ± 8.4 kJ mol−1, respectively), suggesting Ge–Cl bonds can dissociate at a lower temperature than Ge–O bonds.34 We speculate that this could contribute to the formation of Ge NCs at lower temperatures.
The Ge NCs are functionalized using hydrogermylation. Briefly, the annealed GeOX glass is etched with warm water to dissolve GeO2 and liberate freestanding Ge NCs, which are then dispersed in HF acid to terminate Ge NCs with hydrogen atoms.23,24 Finally, the H-terminated Ge NCs are dispersed in 1-octadecene and heated to 200 °C in an inert atmosphere to initiate hydrogermylation, terminating the Ge NC surfaces with 1-octadecene. TEM images of the passivated sample suggest that the GeO2 and precursors are removed (Fig. S16, ESI†). The main lattice spacing in selected area electron diffraction (3.3, 2.0, and 1.7 Å) corresponds to crystalline Ge, confirming the presence of Ge NCs. Fourier transform infrared spectroscopy shows the presence of Ge–C stretching mode, indicating the success of surface passivation (Fig. S17, ESI†).
We attempted to measure photoluminescence (PL) from the hydrogen-terminated and 1-octadecene-terminated samples; however, no PL is detected in any sample. The absence of PL could arise from surface defects, dangling bonds from etching, impurities, or surface species that are not adequately removed during the etching process. The absence of PL is also reported for Ge NCs obtained using similar oxide-based solid-state synthesis.24
In conclusion, we report the synthesis of Ge NCs using solid-state disproportionation of a chloride-derived GeOX glass. XRD and TEM results showed the presence of crystalline Ge particles at processing temperature as low as 325 °C. TEM results also indicated the nanocrystals were highly polydisperse with irregular morphologies. XPS and Raman spectra provided insights into the mechanism by which the NCs were formed, with residual Cl present in the GeOX precursor and Ge with short-range order being formed at temperatures as low as 250 °C. XRD data indicated the GeOX precursor had amorphous features that were shifted to a higher 2θ compared to prior reports. The Ge NCs obtained with this method could be surface-passivated with an organic ligand using hydrogermylation. Compared to other methods for creating Ge NCs, this method takes relatively little time and does not require the use of strong acids or highly exothermic reactions and thus has potential for being scaled up easily, providing a promising route for large-scale synthesis of Ge NCs for various applications. However, better control over size and morphology would be desirable for many applications.
This work was supported by the Air Force Office of Scientific Research Young Investigator Program under grant number FA9550-17-1-0170. MGP acknowledges support from the Herbert L. Stiles faculty fellowship. RDN and BJR acknowledge support from the NSF Graduate Research Fellowship Program (DGE1744592). Support for the Raman spectroscopy measurements was provided by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. The Raman spectroscopy research was performed at the Ames Laboratory, which is operated for the U.S. DOE by Iowa State University under contract # DE-AC02-07CH11358.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. L. Steigerwald, A. P. Alivisatos, J. M. Gibson, T. D. Harris, R. Kortan, A. J. Muller, A. M. Thayer, T. M. Duncan, D. C. Douglass and L. E. Brus, J. Am. Chem. Soc., 1988, 110, 3046–3050 CrossRef CAS.
- A. Mews, A. V. Kadavanich, U. Banin and A. P. Alivisatos, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, R13242 CrossRef CAS.
- A. Cao, Z. Liu, S. Chu, M. Wu, Z. Ye, Z. Cai, Y. Chang, S. Wang, Q. Gong and Y. Liu, Adv. Mater., 2010, 22, 103–106 CrossRef CAS PubMed.
- C. M. Hessel, D. Reid, M. G. Panthani, M. R. Rasch, B. W. Goodfellow, J. Wei, H. Fujii, V. Akhavan and B. A. Korgel, Chem. Mater., 2012, 24, 393–401 CrossRef CAS.
- W. Sun, C. Qian, X. S. Cui, L. Wang, M. Wei, G. Casillas, A. S. Helmy and G. A. Ozin, Nanoscale, 2016, 8, 3678–3684 RSC.
- M. Dasog, G. B. De los Reyes, L. V. Titova, F. A. Hegmann and J. G. C. Veinot, ACS Nano, 2014, 8, 9636–9648 CrossRef CAS PubMed.
- D. C. Lee, J. M. Pietryga, I. Robel, D. J. Werder, R. D. Schaller and V. I. Klimov, J. Am. Chem. Soc., 2009, 131, 3436–3437 CrossRef CAS PubMed.
- D. A. Ruddy, J. C. Johnson, E. R. Smith and N. R. Neale, ACS Nano, 2010, 4, 7459–7466 CrossRef CAS PubMed.
- M. Kanoun, C. Busseret, A. Poncet, A. Souifi, T. Baron and E. Gautier, Solid-State Electron., 2006, 50, 1310–1314 CrossRef CAS.
- Z. C. Holman, C.-Y. Liu and U. R. Kortshagen, Nano Lett., 2010, 10, 2661–2666 CrossRef CAS PubMed.
- Y. J. Cho, H. S. Im, H. S. Kim, Y. Myung, S. H. Back, Y. R. Lim, C. S. Jung, D. M. Jang, J. Park, E. H. Cha, W. I. Cho, F. Shojaei and H. S. Kang, ACS Nano, 2013, 7, 9075–9084 CrossRef CAS PubMed.
- X. Ma, B. Yuan and Z. Yan, Opt. Commun., 2006, 260, 337–339 CrossRef CAS.
- B. R. Taylor, S. M. Kauzlarich, H. W. H. Lee and G. R. Delgado, Chem. Mater., 1998, 10, 22–24 CrossRef CAS.
- X. Ma, F. Wu and S. M. Kauzlarich, J. Solid State Chem., 2008, 181, 1628–1633 CrossRef CAS.
- R. Gresback, Z. Holman and U. Kortshagen, Appl. Phys. Lett., 2007, 91, 093119 CrossRef.
- E. J. Henderson, M. Seino, D. P. Puzzo and G. A. Ozin, ACS Nano, 2010, 4, 7683–7691 CrossRef CAS PubMed.
- M. Hoffman and J. G. C. Veinot, Chem. Mater., 2012, 24, 1283–1291 CrossRef CAS.
- E. J. Henderson, C. M. Hessel and J. G. C. Veinot, J. Am. Chem. Soc., 2008, 130, 3624–3632 CrossRef CAS PubMed.
- X. Lu, K. J. Ziegler, A. Ghezelbash, K. P. Johnston and B. A. Korgel, Nano Lett., 2004, 4, 969–974 CrossRef CAS.
- A. Bernard, K. Zhang, D. Larson, K. Tabatabaei and S. M. Kauzlarich, Inorg. Chem., 2018, 57, 5299–5306 CrossRef CAS PubMed.
- E. Muthuswamy, A. S. Iskandar, M. M. Amador and S. M. Kauzlarich, Chem. Mater., 2013, 25, 1416–1422 CrossRef CAS.
- X. Lu, B. A. Korgel and K. P. Johnston, Chem. Mater., 2005, 17, 6479–6485 CrossRef CAS.
- W. Sun, G. Zhong, C. Kübel, A. A. Jelle, C. Qian, L. Wang, M. Ebrahimi, L. M. Reyes, A. S. Helmy and G. A. Ozin, Angew. Chem., 2017, 129, 6426–6431 CrossRef.
- M. Javadi, D. Picard, R. Sinelnikov, M. A. Narreto, F. A. Hegmann and J. G. C. Veinot, Langmuir, 2017, 33, 8757–8765 CrossRef CAS PubMed.
- M. Javadi, V. K. Michaelis and J. G. C. Veinot, J. Phys. Chem. C, 2018, 122, 17518–17525 CrossRef CAS.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Elsevier, 2012 Search PubMed.
- Z. Xiao, X. Sun, X. Li, Y. Wang, Z. Wang, B. Zhang, X. L. Li, Z. Shen, L. B. Kong and Y. Huang, Nano Lett., 2018, 18, 3290–3296 CrossRef CAS PubMed.
- G. A. Ozin and A. V. Voet, J. Chem. Phys., 1972, 56, 4768–4775 CrossRef CAS.
- D. Bermejo and M. Cardona, J. Non-Cryst. Solids, 1979, 32, 405–419 CrossRef CAS.
- M. Fujii, S. Hayashi and K. Yamamoto, Jpn. J. Appl. Phys., 1991, 30, 687 CrossRef CAS.
- I. H. Campbell and P. M. Fauchet, Solid State Commun., 1986, 58, 739–741 CrossRef CAS.
- A. K. Arora, M. Rajalakshmi, T. R. Ravindran and V. Sivasubramanian, J. Raman Spectrosc., 2007, 38, 604–617 CrossRef CAS.
- A. Schacht, C. Sternemann, A. Hohl, H. Sternemann, C. J. Sahle, M. Paulus and M. Tolan, J. Non-Cryst. Solids, 2009, 355, 1285–1287 CrossRef CAS.
- Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and characterization details. Additional XRD patterns, TEM images, XP, FTIR and Raman spectra. Table of Raman peak assignments and vibrational modes for the GeCl2·dioxane complex. See DOI: 10.1039/c9cc01676g |
| This journal is © The Royal Society of Chemistry 2019 |