Palladium-catalyzed formal insertion of carbenoids into N,O-aminals: direct access to α-alkoxy-β-amino acid esters†
Abstract
A new and efficient reaction has been developed to synthesize α-alkoxy-β-amino acid esters via palladium-catalyzed selective formal insertion of carbenoids into N,O-aminals under mild reaction conditions. A broad range of α-diazoesters with different substituents and various N,O-aminals were compatible with this protocol, affording the corresponding α-alkoxy-β-amino acid esters in good to excellent yields.
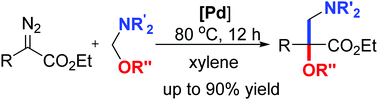


 Please wait while we load your content...
Please wait while we load your content...