Open Access Article
Open Access ArticleSuperhydrophobic states of 2D nanomaterials controlled by atomic defects can modulate cell adhesion†
Manish K.
Jaiswal
 *a,
Kanwar Abhay
Singh
a,
Giriraj
Lokhande
*a,
Kanwar Abhay
Singh
a,
Giriraj
Lokhande
 a and
Akhilesh K.
Gaharwar
a and
Akhilesh K.
Gaharwar
 *abc
*abc
aDepartment of Biomedical Engineering, Texas A&M University, College Station, TX-77843, USA. E-mail: gaharwar@tamu.edu; manish@tamu.edu
bDepartment of Biomedical Engineering, Texas A&M University, College Station, TX-77843, USA
cCenter for Remote Health Technologies & Systems, Texas A&M University, College Station, TX-77843, USA
First published on 7th June 2019
Abstract
We introduced a new concept to the control of wetting characteristics by modulating the degree of atomic defects of two-dimensional transition metal dichalcogenide nanoassemblies of molybdenum disulfide. This work shed new light on the role of atomic vacancies on wetting characteristic that can be leveraged to develop a new class of superhydrophobic surfaces for various applications without altering their topography.
Superhydrophobic materials are used extensively in bioelectronics, medical, and self-cleaning devices. These superhydrophobic properties rely on altering topological features such as surface roughness and surface energy. For instance, microstructural features with grooves filled with air-packets govern the wetting behavior of microengineered superhydrophobic surfaces.1 Such behavior has been explained by Cassie–Baxter wetting theory, where liquid droplets make contact only with tips of the grooves, and intact air-packets support the spherical shape of the droplet to provide superhydrophobic properties.2,3 These methods involve a cumbersome fabrication approach and cannot be applied to biosensing, lab-on-a-chip, blood-repellent, anti-fouling, and self-cleaning applications, which demand a non-textured approach to achieve a superhydrophobic state.
Two-dimensional (2D) nanomaterials have received considerable attention due to high degree of structural anisotropy and chemical functionality.4–7 For example, 2D transition metal dichalcogenide (TMD) nanosheets have been investigated for nanoelectronics, optical sensors, renewable energy sources, catalysis, and lubrication.8–10 In particular, 2D molybdenum disulfide (MoS2) has been explored in nanoelectronics9,11 and as a photothermal agent12–14 due to its tunable band gap. The atomic defects present on the lattice plane of MoS2 provide an active site for various applications, including catalyst and chemical conjugations.10,15 More recently, hierarchical nanoassemblies of defect-rich MoS2 have been shown to improve the performance of lithium ion storage batteries by 20-fold compared with traditional monolayers of MoS2,16–19 and can be used to form hydrogels using polymeric binders via metal-click chemistry.20 MoS2 is cytocompatible and degrades in an in vitro microenvironment and, thus, has been explored for various biomedical applications.21
In the past few years, the superhydrophobic characteristics of 2D nanomaterials such as graphene,22 boron nitride,23 tungsten disulfide (WS2),24 and MoS225 have been reported. The wetting characteristics of these 2D nanomaterials are attributed mostly to the surface roughness or deposition of air-borne hydrocarbons which, ultimately, reduce the surface activity.25,26 Atomic defects that can be incorporated into 2D nanomaterials during the synthetic process have not been investigated but which could provide a facile approach to tune the wetting characteristics.
Here, we report the tunable wetting behavior of defect-rich MoS2via modulating atomic vacancies. Specifically, we demonstrated that MoS2 with a low degree of atomic defects resulted in superhydrophobic behavior (contact angle >150°), whereas a high degree of atomic defects resulted in superhydrophilic properties (contact angle ∼0). The wetting properties controlled by atomic vacancies displayed minimum hysteresis of the contact angle, indicating that the superhydrophobic behavior was independent of the amount of energy dissipated during wetting and dewetting. The superhydrophobic MoS2 could be coated on various substrates (glass, silica, rubber and paper), thereby indicating high versatility for surface-coating applications.
MoS2 nanoassemblies with predefined atomic defects were synthesized by modulating the ratio of precursors of molybdenum (hexaammonium heptamolybdate)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sulfur (thiourea) (1
sulfur (thiourea) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
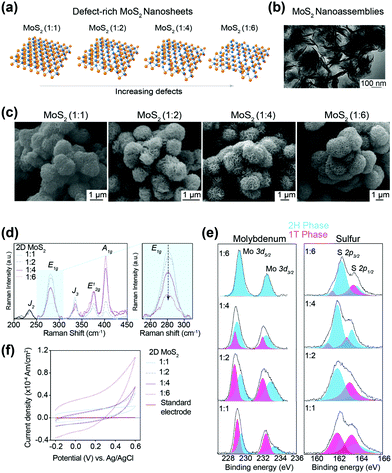
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (see ESI†). The defect-rich MoS2 lattice had hexagonal crystallographic arrangements of Mo and S atoms (Fig. 1a). Depending on the precursor ratio (Mo
6) (see ESI†). The defect-rich MoS2 lattice had hexagonal crystallographic arrangements of Mo and S atoms (Fig. 1a). Depending on the precursor ratio (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S), the number of atomic defects could be modulated from sample 1
S), the number of atomic defects could be modulated from sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. The transmission electron microscopy (TEM) image of typical defect-rich MoS2 shows the nanoassembly of individual 2D sheets (Fig. 1b). The scanning electron microscopy (SEM) images of MoS2 samples with different ratios of Mo and S demonstrated a well-segregated “flower-like” hierarchical architecture (Fig. 1c). The average size of nanoassemblies was 1.5–3 μm. A significant effect of atomic defects on the size of nanoassemblies was not observed (Fig. S1, ESI†).
6. The transmission electron microscopy (TEM) image of typical defect-rich MoS2 shows the nanoassembly of individual 2D sheets (Fig. 1b). The scanning electron microscopy (SEM) images of MoS2 samples with different ratios of Mo and S demonstrated a well-segregated “flower-like” hierarchical architecture (Fig. 1c). The average size of nanoassemblies was 1.5–3 μm. A significant effect of atomic defects on the size of nanoassemblies was not observed (Fig. S1, ESI†).
In 2D MoS2, a layer of Mo (oxidative state, 4+) atoms was held between two layers of S (oxidative state, 2−) atoms. Two MoS2 layers interacted with each other via short-range van der Waals interactions. The Mo atoms could have trigonal prismatic co-ordination with S atoms to result in hexagonal symmetry (2H phase) or octahedral co-ordination to result in tetragonal symmetry (1T phase). The Raman spectra showed the formation of a dominant 2H phase with partial presence of the 1T phase (Fig. 1d). The characteristic E12g (in-plane vibration of two S atoms) and A1g (out-of-plane vibration of S atoms) occurred at ∼375 cm−1 and ∼402 cm−1, respectively. The appearance of the E1g peak at 280 cm−1 (which was due to the tetragonal symmetry of the lattice) indicated a metastable 1T phase besides the dominant 2H phase in MoS2 samples, which was likely due to ammonium cations released during synthesis.27 Furthermore, as expected, a simultaneous attenuation in the intensity of the E1g peak accompanied with gradual amplification in E12g and A1g from sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to sample 1
1 to sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 demonstrated the formation of a highly ordered 2H phase with an increase in thiourea concentration. The observation in Raman spectra was corroborated further with X-ray photoelectron spectroscopy (XPS) for the core-level binding energies of Mo and S atoms for MoS2 samples (1
6 demonstrated the formation of a highly ordered 2H phase with an increase in thiourea concentration. The observation in Raman spectra was corroborated further with X-ray photoelectron spectroscopy (XPS) for the core-level binding energies of Mo and S atoms for MoS2 samples (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (Fig. 1e). The presence of the 1T phase (tetragonal symmetry) was dominated by the 2H phase (hexagonal symmetry), as represented by blue curves with increments in the sulfur precursor (thiourea content) from 1
6) (Fig. 1e). The presence of the 1T phase (tetragonal symmetry) was dominated by the 2H phase (hexagonal symmetry), as represented by blue curves with increments in the sulfur precursor (thiourea content) from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 through 1
1 through 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. These results corroborated the findings of Raman spectroscopy. The specific BE for the 1T-phase Mo was (3d5/2) 229.2 and (3d3/2) 232.4 eV, and for S was (2p3/2) 161.9 and (2p1/2) 163.1 eV. The corresponding BE for the 2H phase was slightly higher for Mo (229.6 and 233.3 eV) and S (162.2 eV and 163.5 eV), respectively.
6. These results corroborated the findings of Raman spectroscopy. The specific BE for the 1T-phase Mo was (3d5/2) 229.2 and (3d3/2) 232.4 eV, and for S was (2p3/2) 161.9 and (2p1/2) 163.1 eV. The corresponding BE for the 2H phase was slightly higher for Mo (229.6 and 233.3 eV) and S (162.2 eV and 163.5 eV), respectively.
The number of atomic vacancies for 2D MoS2 was determined with cyclic voltammetry via expanded J/V (current density vs. potential) curves (Fig. 1f). An increase in the sulfur precursor (thiourea) resulted in an increase in the area under the J/V curve. The concentration of atomic defects increased with an increase in the Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S precursor ratio, 1
S precursor ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (∼0.59 × 102 μM g−1), 1
1 (∼0.59 × 102 μM g−1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (∼0.72 × 102 μM g−1), 1
2 (∼0.72 × 102 μM g−1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (∼0.80 × 102 μM g−1) and 1
4 (∼0.80 × 102 μM g−1) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (∼2.1 × 102 μM g−1) (see ESI† for details).
6 (∼2.1 × 102 μM g−1) (see ESI† for details).
The effect of atomic vacancy on wetting characteristics was evaluated by determining the contact angle of water and polar/non-polar organic solvents on MoS2-coated surfaces. To determine wetting characteristics, the water contact angle on a MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
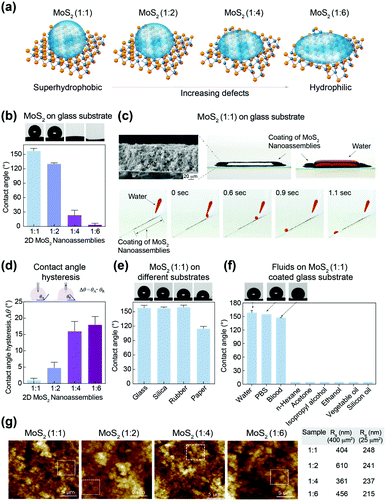
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)-coated glass substrate was evaluated (Fig. 2a). The highest contact angle was observed for sample 1
6)-coated glass substrate was evaluated (Fig. 2a). The highest contact angle was observed for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (157.5 ± 5.2°), which decreased gradually for sample 1
1 (157.5 ± 5.2°), which decreased gradually for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (137.6 ± 2.0°), sample 1
2 (137.6 ± 2.0°), sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (23.7 ± 10.0°) and sample 1
4 (23.7 ± 10.0°) and sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (3.6 ± 3.0°), thus causing the surface to become superhydrophilic. The associated liquid-droplet images are shown on top of the bar graph. As expected, the droplet shape was spherical for 1
6 (3.6 ± 3.0°), thus causing the surface to become superhydrophilic. The associated liquid-droplet images are shown on top of the bar graph. As expected, the droplet shape was spherical for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating superhydrophobic characteristics. As the ratio of sulfur precursor increased during MoS2 synthesis, the water droplet became oblate (1
1, indicating superhydrophobic characteristics. As the ratio of sulfur precursor increased during MoS2 synthesis, the water droplet became oblate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and eventually spread (1
2) and eventually spread (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and adsorbed (1
4) and adsorbed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) onto the coated surface, thereby suggesting atomic defect-dependent wetting behavior.
6) onto the coated surface, thereby suggesting atomic defect-dependent wetting behavior.
To further verify superhydrophobic characteristics, a rectangular pattern (2 cm × 1 cm) covering the boundary area using MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the glass slide was filled with 1 mL of water (Fig. 2b). The uniform coating of MoS2 (1
1) on the glass slide was filled with 1 mL of water (Fig. 2b). The uniform coating of MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the glass substrate was verified using SEM. The rectangular pattern (thickness, 0.5 mm) could hold 10-fold water molecules (height, 5 mm) due to its superhydrophobic characteristics. Even after it was subjected to manual shaking, the movement of water was confined within the rectangle area due to the hydrophobic nature of the MoS2 (see ESI,† Video V1). In addition, a water droplet was allowed to roll-down a slanted glass slide coated with MoS2 (1
1) on the glass substrate was verified using SEM. The rectangular pattern (thickness, 0.5 mm) could hold 10-fold water molecules (height, 5 mm) due to its superhydrophobic characteristics. Even after it was subjected to manual shaking, the movement of water was confined within the rectangle area due to the hydrophobic nature of the MoS2 (see ESI,† Video V1). In addition, a water droplet was allowed to roll-down a slanted glass slide coated with MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to investigate the superhydrophobic properties in dynamic conditions. The water droplet (red) rolled down without being adsorbed over the surface and remained intact (spherical shape) during rolling. Hence, MoS2 (1
1) to investigate the superhydrophobic properties in dynamic conditions. The water droplet (red) rolled down without being adsorbed over the surface and remained intact (spherical shape) during rolling. Hence, MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be used for various coating applications (Fig. 2b and c; see ESI,† Video V2).
1) could be used for various coating applications (Fig. 2b and c; see ESI,† Video V2).
The wetting behavior of a MoS2-coated surface can be understood in terms of the interfacial tension (γ) between the liquid and the coating as given by Young's equation,28 cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = (γsa − γsl)/γla, where θ is the contact angle, γsl is the solid/liquid interfacial free energy, γsa is the solid surface free energy, and γla is the liquid surface free energy. As the Mo
θ = (γsa − γsl)/γla, where θ is the contact angle, γsl is the solid/liquid interfacial free energy, γsa is the solid surface free energy, and γla is the liquid surface free energy. As the Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S precursor ratio changed from 1
S precursor ratio changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the interaction between the MoS2-coated surface and water molecules increased due to the presence of defects owing to direct access of the Mo atomic plane, and could potentially form a molybdenum–water complex. This resulted in reduction of the interfacial tension between the liquid and substrate (γsl) and surface tensions (γsa and γla) approached each other. This results in θ tended to zero, thus causing the sample to become superhydrophilic.
6, the interaction between the MoS2-coated surface and water molecules increased due to the presence of defects owing to direct access of the Mo atomic plane, and could potentially form a molybdenum–water complex. This resulted in reduction of the interfacial tension between the liquid and substrate (γsl) and surface tensions (γsa and γla) approached each other. This results in θ tended to zero, thus causing the sample to become superhydrophilic.
The contact angle is often a function of whether the liquid is advancing or receding on the substrate.28 To ascertain such possible behavior of the samples, the contact angle hysteresis (Δθ) was measured (which is essentially the difference between advancing (θA) and receding contact angles (θR)). As expected, Δθ was minimal for the MoS2 samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1 ± 0.6°) and 1
1 (1 ± 0.6°) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (4.8 ± 1.7°), indicating that their superhydrophobic character was independent of roughness or surface contamination (Fig. 2d). Higher Δθ was observed for sample 1
2 (4.8 ± 1.7°), indicating that their superhydrophobic character was independent of roughness or surface contamination (Fig. 2d). Higher Δθ was observed for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (16 ± 3°) and 1
4 (16 ± 3°) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (18 ± 2.5°). The low Δθ for MoS2 (1
6 (18 ± 2.5°). The low Δθ for MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) indicated that the Cassi–Baxter wetting behavior of the liquid caused the droplets to roll-down in a spherical shape over the slightly slanted surface (see ESI†). Such superhydrophobic materials with small Δθ are highly sought after for commercial applications (e.g., designing self-cleaning and spill-resistance coating materials for exterior glass and wind shields).
1) indicated that the Cassi–Baxter wetting behavior of the liquid caused the droplets to roll-down in a spherical shape over the slightly slanted surface (see ESI†). Such superhydrophobic materials with small Δθ are highly sought after for commercial applications (e.g., designing self-cleaning and spill-resistance coating materials for exterior glass and wind shields).
To further investigate the versatility of MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a superhydrophobic coating, substrates such as silica, rubber and papers were coated with MoS2 (1
1) as a superhydrophobic coating, substrates such as silica, rubber and papers were coated with MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the water contact angle determined (Fig. 2e). All the surfaces showed superhydrophobic characteristics: silica (157.8 ± 5.2°), rubber (159.7 ± 4.0°), and paper (115 ± 4.7°). In addition, the contact angle for various biological fluids and organic solvents, including phosphate-buffered saline (PBS) (pH 7.4), whole blood, hexane, ethanol, isopropanol, fatty vegetable oil and silicone oil, were investigated (Fig. 2f). The contact angle for PBS (151.0 ± 1.07°) and whole blood (147.2 ± 0.41°) was similar to that of water. For glycerol (106.0 ± 28.6°), silicone oil (16.4 ± 2.5°) and other organic solvents (∼5°), the contact angle was lower due to enhanced interactions of the sulfur layer (from MoS2) with hydrocarbons, acetone and mild polar alcohols. Moreover, an aqueous solution of an amphiphile, such as sodium dodecyl sulfate (SDS), spread onto the MoS2 (1
1) and the water contact angle determined (Fig. 2e). All the surfaces showed superhydrophobic characteristics: silica (157.8 ± 5.2°), rubber (159.7 ± 4.0°), and paper (115 ± 4.7°). In addition, the contact angle for various biological fluids and organic solvents, including phosphate-buffered saline (PBS) (pH 7.4), whole blood, hexane, ethanol, isopropanol, fatty vegetable oil and silicone oil, were investigated (Fig. 2f). The contact angle for PBS (151.0 ± 1.07°) and whole blood (147.2 ± 0.41°) was similar to that of water. For glycerol (106.0 ± 28.6°), silicone oil (16.4 ± 2.5°) and other organic solvents (∼5°), the contact angle was lower due to enhanced interactions of the sulfur layer (from MoS2) with hydrocarbons, acetone and mild polar alcohols. Moreover, an aqueous solution of an amphiphile, such as sodium dodecyl sulfate (SDS), spread onto the MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)-coated glass substrate.
6)-coated glass substrate.
To investigate the effect of surface roughness on wetting characteristics, topographical analysis was carried out using atomic force microscopy (AFM). The root-mean square value of roughness (Rq) was calculated for an imaging area of 400 and 25 μm2 for all samples (Fig. 2g). There was no significant difference in Rq (400–600 nm for 400 μm2 and 220–250 nm for 25 μm2) for different MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) samples. This observation suggested that topographical features did not govern the hydrophobic character of MoS2.
6) samples. This observation suggested that topographical features did not govern the hydrophobic character of MoS2.
We investigated the adhesion of human mesenchymal stem cells (hMSCs) onto superhydrophobic (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and superhydrophilic surfaces (1
1) and superhydrophilic surfaces (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
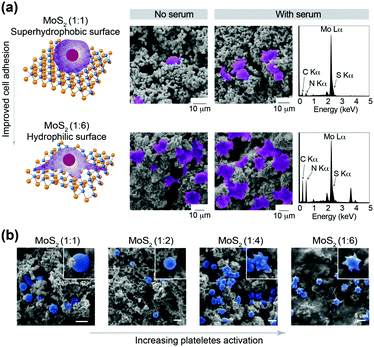
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). High adhesion of hMSCs was observed on hydrophilic surfaces. Superhydrophobic surfaces of MoS2 (1
6). High adhesion of hMSCs was observed on hydrophilic surfaces. Superhydrophobic surfaces of MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) restricted adhesion of hMSCs compared with MoS2 (1
1) restricted adhesion of hMSCs compared with MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (Fig. 3a). To investigate the role of protein adsorption, we tested adhesion of hMSCs in the presence and absence of serum. We observed significantly higher adhesion of hMSCs in the presence of serum in both MoS2 surfaces (1
6) (Fig. 3a). To investigate the role of protein adsorption, we tested adhesion of hMSCs in the presence and absence of serum. We observed significantly higher adhesion of hMSCs in the presence of serum in both MoS2 surfaces (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). Energy-dispersive X-ray spectroscopy (EDS) mapping for C and N elements confirmed the higher adhesion of hMSCs on hydrophilic surfaces of MoS2 (1
6). Energy-dispersive X-ray spectroscopy (EDS) mapping for C and N elements confirmed the higher adhesion of hMSCs on hydrophilic surfaces of MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). However, we did not observe a significant change in protein adsorption on MoS2 surfaces (1
6). However, we did not observe a significant change in protein adsorption on MoS2 surfaces (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). We further evaluated the effect of wetting characteristics on platelet activation. The adhesion and activation of platelets was done using bovine blood on MoS2 surfaces (1
6). We further evaluated the effect of wetting characteristics on platelet activation. The adhesion and activation of platelets was done using bovine blood on MoS2 surfaces (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 through 1
1 through 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (Fig. 3b). The hydrophilic nature of the surface promoted platelet activation.
6) (Fig. 3b). The hydrophilic nature of the surface promoted platelet activation.
In summary, we introduced a new concept to control the wetting characteristics of 2D nanomaterials by modulating the degree of atomic defects. Specifically, we synthesized superhydrophobic 2D MoS2 and demonstrated that atomic defects dictated hydrophilic-to-hydrophobic transition. The highest contact angle was observed for MoS2 with a Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S precursor ratio of 1
S precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (157.5 ± 5.2°), which decreased gradually for 1
1 (157.5 ± 5.2°), which decreased gradually for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (137.6 ± 2.0°), 1
2 (137.6 ± 2.0°), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (23.7 ± 10.0°) and 1
4 (23.7 ± 10.0°) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (3.6 ± 3.0°), thus causing the surface to become superhydrophilic. MoS2 (1
6 (3.6 ± 3.0°), thus causing the surface to become superhydrophilic. MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be coated readily on glass, silica, rubber and paper and retain its superhydrophobic characteristics. Due to low Δθ, superhydrophobic MoS2 (1
1) could be coated readily on glass, silica, rubber and paper and retain its superhydrophobic characteristics. Due to low Δθ, superhydrophobic MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be used for biosensing, lab-on-a-chip, blood-repellent, anti-fouling, and self-cleaning applications, which demand a non-textured approach to achieve a superhydrophobic state. The wetting characteristics of the MoS2 surface directly influence cell-adhesion characteristics that can be leveraged for biomedical applications.
1) could be used for biosensing, lab-on-a-chip, blood-repellent, anti-fouling, and self-cleaning applications, which demand a non-textured approach to achieve a superhydrophobic state. The wetting characteristics of the MoS2 surface directly influence cell-adhesion characteristics that can be leveraged for biomedical applications.
We thank James L. Gentry for the synthesis and deposition of defect-rich MoS2 films and preparing supporting videos. Dr James K. Carrow and Dr Jagriti Gupta (IIT Bombay) assisted in contact-angle measurements and cyclic voltammetry, respectively. The research reported in this publication was supported by the National Institutes of Health (NIH) Director's New Innovator Award (DP2 EB026265) by National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. Jokinen, E. Kankuri, S. Hoshian, S. Franssila and R. H. A. Ras, Adv. Mater., 2018, 30, 1705104 CrossRef PubMed.
- F.-M. Chang, S.-J. Hong, Y.-J. Sheng and H.-K. Tsao, Appl. Phys. Lett., 2009, 95, 064102 CrossRef.
- J. López-Cabrelles, S. Mañas-Valero, I. J. Vitórica-Yrezábal, P. J. Bereciartua, J. A. Rodríguez-Velamazán, J. C. Waerenborgh, B. J. C. Vieira, D. Davidovikj, P. G. Steeneken, H. S. J. van der Zant, G. Mínguez Espallargas and E. Coronado, Nat. Chem., 2018, 10, 1001–1007 CrossRef PubMed.
- D. Voiry, A. Goswami, R. Kappera, E. SilvaCecilia de Carvalho Castro, D. Kaplan, T. Fujita, M. Chen, T. Asefa and M. Chhowalla, Nat. Chem., 2015, 7, 45–49 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1226419 CrossRef.
- A. K. Gaharwar, L. M. Cross, C. W. Peak, K. Gold, J. K. Carrow, A. Brokesh and K. A. Singh, Adv. Mater., 2019 DOI:10.1002/adma.201900332.
- D. Chimene, D. Alge and A. K. Gaharwar, Adv. Mater., 2015, 27, 7261–7284 CrossRef CAS PubMed.
- W. Li, X. Gao, D. Xiong, F. Wei, W.-G. Song, J. Xu and L. Liu, Adv. Energy Mater., 2017, 7, 1602579 CrossRef.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- T. Liu, S. Shi, C. Liang, S. Shen, L. Cheng, C. Wang, X. Song, S. Goel, T. E. Barnhart, W. Cai and Z. Liu, ACS Nano, 2015, 9, 950–960 CrossRef CAS PubMed.
- C. N. R. Rao, H. S. S. Ramakrishna Matte and U. Maitra, Angew. Chem., Int. Ed., 2013, 52, 13162–13185 CrossRef CAS PubMed.
- W. Yin, L. Yan, J. Yu, G. Tian, L. Zhou, X. Zheng, X. Zhang, Y. Yong, J. Li, Z. Gu and Y. Zhao, ACS Nano, 2014, 8, 6922–6933 CrossRef CAS PubMed.
- M. Li, A. Zhao, K. Dong, W. Li, J. Ren and X. Qu, Nano Res., 2015, 8, 3216–3227 CrossRef CAS.
- W. Yin, J. Yu, F. Lv, L. Yan, L. R. Zheng, Z. Gu and Y. Zhao, ACS Nano, 2016, 10, 11000–11011 CrossRef CAS PubMed.
- S. S. Chou, N. Sai, P. Lu, E. N. Coker, S. Liu, K. Artyushkova, T. S. Luk, B. Kaehr and C. J. Brinker, Nat. Commun., 2015, 6, 8311 CrossRef CAS PubMed.
- G. Ye, Y. Gong, J. Lin, B. Li, Y. He, S. T. Pantelides, W. Zhou, R. Vajtai and P. M. Ajayan, Nano Lett., 2016, 16, 1097–1103 CrossRef CAS PubMed.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- Z. Wu, B. Li, Y. Xue, J. Li, Y. Zhang and F. Gao, J. Mater. Chem. A, 2015, 3, 19445–19454 RSC.
- M. Wang, G. Li, H. Xu, Y. Qian and J. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 1003–1008 CrossRef CAS PubMed.
- M. K. Jaiswal, J. K. Carrow, J. L. Gentry, J. Gupta, N. Altangerel, M. Scully and A. K. Gaharwar, Adv. Mater., 2017, 29, 1702037 CrossRef PubMed.
- R. Kurapati, L. Muzi, A. P. R. de Garibay, J. Russier, D. Voiry, I. A. Vacchi, M. Chhowalla and A. Bianco, Adv. Funct. Mater., 2017, 27, 1605176 CrossRef.
- J. Rafiee, M. A. Rafiee, Z. Z. Yu and N. Koratkar, Adv. Mater., 2010, 22, 2151–2154 CrossRef CAS PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- R. Xu, K. Zhang, X. Xu, M. He, F. Lu and B. Su, Adv. Sci., 2018, 5, 1700655 CrossRef PubMed.
- J. Choi, J. Mun, M. C. Wang, A. Ashraf, S.-W. Kang and S. Nam, Nano Lett., 2017, 17, 1756–1761 CrossRef CAS PubMed.
- A. Kozbial, X. Gong, H. Liu and L. Li, Langmuir, 2015, 31, 8429–8435 CrossRef CAS PubMed.
- Y. Jiao, A. Mukhopadhyay, Y. Ma, L. Yang, A. M. Hafez and H. Zhu, Adv. Energy Mater., 2018, 8, 1702779 CrossRef.
- F.-M. Chang, S.-J. Hong, Y.-J. Sheng and H.-K. Tsao, Appl. Phys. Lett., 2009, 95, 064102 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods and materials characterizations are detailed. See DOI: 10.1039/c9cc00547a |
| This journal is © The Royal Society of Chemistry 2019 |