Thiourea dioxide as a source of sulfonyl groups: photoredox generation of sulfones and sulfonamides from heteroaryl/aryl halides†
Shengqing
Ye‡
a,
Yuewen
Li‡
 b,
Jie
Wu
b,
Jie
Wu
 *abc and
Zhiming
Li
*abc and
Zhiming
Li
 *b
*b
aInstitute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou 318000, China. E-mail: jie_wu@fudan.edu.cn
bDepartment of Chemistry, Fudan University, 2005 Songhu Road, Shanghai 200438, China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 4th February 2019
Abstract
Thiourea dioxide as the source of sulfonyl groups for the efficient synthesis of heteroaryl sulfones and sulfonamides from heteroaryl halides under visible light irradiation is reported. This transformation proceeds smoothly via heteroaryl sulfinate intermediates, which can be trapped in situ by various electrophiles. A broad reaction scope is demonstrated, especially for the electron-deficient heteroaryl halides. Mechanistic studies show that the radical coupling of the heteroaryl radical and sulfur dioxide radical anion may be the key step during the reaction process, as supported by EPR spectroscopy and DFT calculations.
It is well-known that the applications of sulfonyl-containing compounds in pharmaceuticals,1 agrochemicals,2 and materials3 have been demonstrated. Additionally, heteroaryl sulfones and sulfonamides have displayed excellent biological activities.4 The traditional method for the preparation of sulfones is through the sulfonylation of arenes or oxidation of sulfides.5 The general route to sulfonamides is from the condensation of sulfonyl chlorides and amines.6 Thus, odorous thiol compounds and chlorosulfonic acid are often required, and the substrate scope is limited due to the harsh reaction conditions. Considering the importance of sulfonyl-containing compounds, it is highly desirable to provide a general and simple method for the formation of sulfones and sulfonamides under mild conditions.
Recently, the insertion of sulfur dioxide into organic molecules for the synthesis of sulfonyl compounds has attracted much attention. The bench-stable DABCO·(SO2)27,8a and inorganic sulfites8b have been applied successfully as the sulfur dioxide surrogates avoiding the use of gaseous sulfur dioxide. The transformations could proceed under transition metal catalysis or through radical processes. So far, aryl halides,8 arylboronic acids,9 aryl nonaflates,10 and aryl triethoxysilanes11 have been proven to be good partners in the metal-catalyzed reactions with the insertion of sulfur dioxide. Additionally, aryl or heteroaryl halides with a stoichiometric amount of metal could be converted to the corresponding organometallic reagents, which would act as a nucleophile to react with sulfur dioxide giving rise to sulfinates.12 The metal sulfinates would subsequently undergo electrophilic reaction to afford sulfonyl compounds. Interestingly, the carbon radicals formed in situ from different precursors13–19 would be captured by sulfur dioxide, leading to the corresponding sulfonyl radicals. These sulfonyl radicals would further react with unsaturated bonds or metal intermediates providing various sulfonyl compounds.
Although significant progress for the synthesis of sulfonyl compounds from sulfur dioxide has been witnessed,20 there are few reports on the sulfonation of electron-deficient heterocycles via insertion of sulfur dioxide.11d,21 To our knowledge, it is hard for a carbon radical with a heteroatom in its 2-position to capture sulfur dioxide, thus providing a sulfonyl radical.22 Recently, we described the sulfonylation of (hetero)aryl iodides by using sodium dithionite as the sulfonyl source.21b We conceived that the sulfonyl source might be broadened to others, and it would be attractive and challenging to develop a route for access to sulfonated heterocyclic compounds through radical coupling procedures.
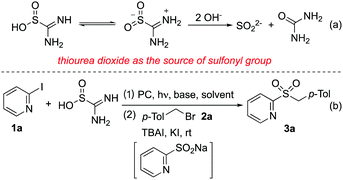
Thiourea dioxide has been widely used as a strong reducing agent in various transformations.23 In the presence of hydroxide, thiourea dioxide would be converted to sulfur dioxide anions and urea (Scheme 1, eqn (a)). We envisioned that under suitable conditions, sulfur dioxide anions would undergo a single electron transfer (SET) thus providing the source of the sulfonyl group. Herein, we report that for the first time, the cheap and commercially available thiourea dioxide is unprecedently applied as the source of sulfonyl groups for the efficient synthesis of heteroaryl sulfones and sulfonamides from heteroaryl halides under visible light irradiation. This transformation proceeds smoothly via heteroaryl sulfinate intermediates, which can be trapped in situ by various electrophiles. A broad reaction scope is demonstrated, especially for the electron-deficient heteroaryl halides. Mechanistic studies show that the radical coupling of heteroaryl radicals and sulfur dioxide radical anions may be the key step during the reaction process.
 | ||
| Scheme 1 Initial studies for the reaction of 2-iodopyridine 1a, thiourea dioxide and 1-(bromomethyl)-4-methylbenzene 2a. | ||
Since thiourea dioxide would be converted to sulfur dioxide anions in the presence of hydroxide, heteroaryl halides were selected as substrates for reaction development. We expected that sulfur dioxide anions would be transferred to sulfur dioxide radical anions under suitable conditions, which would react with heteroaryl halides via a single electron transfer (SET) to produce heteroaryl sulfinates. To test the above hypothesis, a reaction of 2-iodopyridine 1a and thiourea dioxide was selected as the model for initial studies (Scheme 1, eqn (b). For details, please see the ESI†). As mentioned above, thiourea dioxide showed reducibility in alkaline conditions, so at the outset the reaction was performed at room temperature in several solvents in the presence of NaOH (Table S1, entries 1–6, ESI†). Gratifyingly, the formation of sodium 2-pyridinesulfinate was observed when the reaction occurred in DMF, DMA or DMSO. Subsequently, TBAI, KI and 1-(bromomethyl)-4-methylbenzene 2a were added in these reactions. It was found that the expected product 3a could be afforded in 42% isolated yield in DMSO (Table S1, entry 6, ESI†). The yield of product 3a was increased to 80% when the reaction was stirred under visible-light irradiation with Ir(ppy)3 (Table S1, entry 8, ESI†). After screening other photocatalysts (Table S1, entries 9–13, ESI†), we found that the cheap fluorescein could replace the expensive iridium catalyst with comparative yield (Table S1, entry 13, ESI†). No better results were obtained when other bases were used in the reaction (Table S1, entries 14–17, ESI†). Changing the amount of thiourea dioxide or NaOH could not improve the final outcome (Table S1, entries 18 and 19, ESI†).
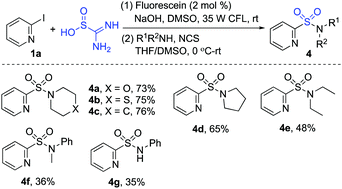
The scope of the reaction of heteroaryl halides 1, thiourea dioxide, and 1-(bromomethyl)-4-methylbenzene 2a was then explored. As shown in Scheme 2, a range of heteroaryl iodides 1 were suitable substrates under these conditions. Since the less active heteroaryl-bromides are much cheaper than heteroaryl-iodides, we further examined the reaction of heteroaryl/aryl bromides. Although only a trace amount of product was detected when 2-bromopyridine was used as the substrate under the standard conditions, product 3a could be obtained in 63% yield when Ir(ppy)3 was used as the photocatalyst instead of fluorescein. Many functional groups including ester, trifluoromethyl, chloro, bromo, amino, and hydroxy were tolerated. Reactions of other heteroaryl halides as substrates were further explored. It showed that examples of pyrazine, pyrimidine, quinoline, isoquinoline and thiophene ring systems were all proven to be applicable, and the corresponding sulfones were produced as expected. However, the result was not satisfactory when 1-iodo-4-nitrobenzene was employed in the reaction. We postulated that the aryl radical generated in situ would be hydrogenated easily.24 To verify the usefulness of this sulfonation method, the reaction of thiourea dioxide and 1-(bromomethyl)-4-methylbenzene 2a was scaled up by using 1.0 gram of 2-iodopyridine 1a. The procedure proceeded smoothly, affording the desired product 3a in 78% yield.
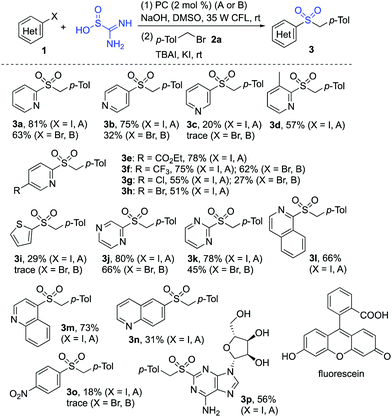 | ||
| Scheme 2 Reactions of heteroaryl/aryl iodides 1, thiourea dioxide and 1-(bromomethyl)-4-methylbenzene 2a. (Isolated yield based on heteroaryl/aryl iodide 1. A: fluorescein; B: Ir(ppy)3). | ||
Next, we examined the reactions by using other alkyl bromides as electrophilic substrates in the reaction of 2-iodopyridine 1a with thiourea dioxide. The result is shown in Scheme 3. As expected, all reactions worked well to provide the corresponding products.
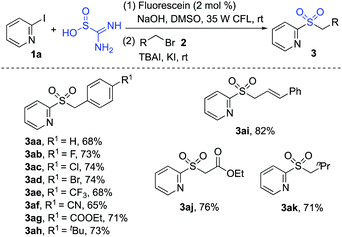 | ||
| Scheme 3 Reactions of 2-iodopyridine 1a, thiourea dioxide and other alkyl bromides 2 (isolated yield based on 2-iodopyridine 1a). | ||
We further applied this method in the preparation of heteroaryl sulfonamides (Scheme 4). Several aliphatic amines were added in the reaction instead of alkyl bromides in the presence of N-chlorosuccinimide, and the corresponding heterocyclic sulfonamides were obtained in good yields. Additionally, aromatic amines were also compatible in this transformation, although the yields were not satisfactory. We reasoned that the less nucleophilic anilines could not convert smoothly into the corresponding amine chloride during the reaction process.
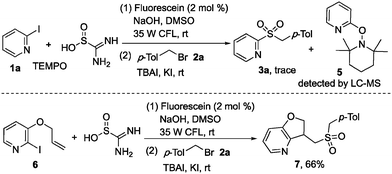
In order to understand the mechanism of this photoinduced reaction, a preliminary mechanistic investigation was carried out. The reaction was hampered when 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) was added in the reaction of 2-iodopyridine 1a, thiourea dioxide, and 1-(bromomethyl)-4-methylbenzene 2a, and the formation of 2-(TEMPO) pyridine 5 was detected by LC-MS (Scheme 5). Meanwhile, compound 7 was obtained in 66% yield when 3-(allyloxy)-2-iodopyridine 6 was employed in the reaction of thiourea dioxide with 1-(bromomethyl)-4-methylbenzene 2a (Scheme 5). These results demonstrated that a heteroaryl radical might be involved in the reaction process.
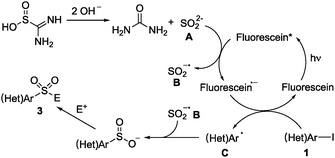
Additionally, a series of control experiments were conducted by using electron paramagnetic resonance (EPR) spectroscopy and a fluorescence spectrophotometer, as shown in the ESI.† The results also demonstrated the radical process of this transformation. Thus, a plausible mechanism of this visible-light induced sulfonylation reaction was proposed, as illustrated in Scheme 6. We reasoned that sulfur dioxide anion A would be generated from the reaction of thiourea dioxide with sodium hydroxide. Subsequently, the excited state of fluorescein would react with sulfur dioxide anion A giving rise to sulfur dioxide radical anion B, along with the formation of a fluorescein radical anion. Then, the fluorescein radical anion would undergo a single electron transfer (SET) with heteroaryl iodide, leading to a heteroaryl radical and an iodide anion. Finally, the heteroaryl sulfinate intermediate would be formed via a radical coupling between sulfur dioxide radical anion B and heteroaryl radical C. This key step in the transformation was supported by DFT calculations in the meantime (see the ESI†).
In conclusion, we have reported that the cheap and commercially available thiourea dioxide is applied as the source of sulfonyl groups for the efficient synthesis of heteroaryl sulfones and sulfonamides from heteroaryl halides under visible light irradiation. This transformation proceeds smoothly via heteroaryl sulfinate intermediates, which can be trapped in situ by various electrophiles. A broad reaction scope is demonstrated, especially for the electron-deficient heteroaryl halides. Mechanistic studies show that the radical coupling of heteroaryl radicals and sulfur dioxide radical anions may be the key step during the reaction process, as supported by EPR spectroscopy and DFT calculations.
Financial support from the National Natural Science Foundation of China (No. 21672037 and 21532001) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) G. H. Whitham, Organosulfur Chemistry, Oxford University, New York, 1995 Search PubMed; (b) G. Liu, J. T. Link, Z. Pei, E. B. Reilly, S. Leitza, B. Nguyen, K. C. Marsh, G. F. Okasinski, T. W. von Geldern, M. Ormes, K. Fowler and M. Gallatin, J. Med. Chem., 2000, 43, 4025 CrossRef CAS PubMed; (c) J. Drews, Science, 2000, 287, 1960 CrossRef CAS PubMed; (d) R. Bentley, Chem. Soc. Rev., 2005, 34, 609 RSC; (e) Y. Harrak, G. Casula, J. Basset, G. Rosell, S. Plescia, D. Raffa, M. G. Cusimano, R. Pouplana and M. D. Pujol, J. Med. Chem., 2010, 53, 6560 CrossRef CAS PubMed.
- (a) M. C. Carreno, Chem. Rev., 1995, 95, 1717 CrossRef CAS; (b) W.-M. Xu, F.-F. Han, M. He, D.-Y. Hu, J. He, S. Yang and B.-A. Song, J. Agric. Food Chem., 2012, 60, 1036 CrossRef CAS PubMed.
- (a) M. Kakimoto, S. J. Grunzinger and T. Hayakawa, Polym. J., 2010, 42, 697 CrossRef CAS; (b) H. Sasabe, Y. Seino, M. Kimura and J. Kido, Chem. Mater., 2012, 24, 1404 CrossRef CAS.
- C. Santelli-Rouvier, J.-M. Barret, C. M. Farrell, D. Sharples, B. T. Hill and J. Barbe, Eur. J. Med. Chem., 2004, 39, 1029 CrossRef CAS PubMed.
- For recent examples, see: (a) L.-Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Org. Chem. Front., 2018, 5, 2604 RSC; (b) L.-Y. Xie, S. Peng, L.-L. Jiang, X. Peng, W. Xia, X. Yu, X.-X. Wang, Z. Cao and W.-M. He, Org. Chem. Front., 2019, 2, 167 RSC; (c) L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X. Yu, N.-N. Dai, Z.-L. Tang, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976 CrossRef CAS; (d) J. Zhu, W.-C. Yang, X.-D. Wang and L. Wu, Adv. Synth. Catal., 2018, 360, 386 CrossRef CAS.
- (a) D. K. Louie, Handbook of sulfuric acid manufacturating, DKL, Thornhill, Ontario, Canada, 1961 Search PubMed; (b) S. W. Wright and K. N. Hallstrom, J. Org. Chem., 2006, 71, 1080 CrossRef CAS PubMed; (c) G. K. S. Prakash, T. Mathew and G. A. Olah, J. Org. Chem., 2007, 72, 5847 CrossRef CAS PubMed; (d) K. Bahrami, M. M. Khodaei and D. Khaledian, Tetrahedron Lett., 2012, 53, 354 CrossRef CAS.
- P. S. Santos and M. T. S. Mello, J. Mol. Struct., 1988, 178, 121 CrossRef CAS.
- (a) B. Nguyen, E. J. Emmet and M. C. Willis, J. Am. Chem. Soc., 2010, 132, 16372 CrossRef CAS PubMed; (b) S. Ye and J. Wu, Chem. Commun., 2012, 48, 10037 RSC; (c) C. S. Richards-Taylor, D. C. Blakemore and M. C. Willis, Chem. Sci., 2014, 5, 222 RSC; (d) H. Konishi, H. Tanaka and K. Manabe, Org. Lett., 2017, 19, 1578 CrossRef CAS PubMed; (e) A. L. Tribby, I. Rodríguez, S. Shariffudin and N. D. Ball, J. Org. Chem., 2017, 82, 2294 CrossRef CAS PubMed; (f) D. Yang, P. Sun, W. Wei, F. Liu, H. Zhang and H. Wang, Chem. – Eur. J., 2018, 24, 4423 CrossRef CAS PubMed.
- (a) S. Ye and J. Wu, Chem. Commun., 2012, 48, 7753 RSC; (b) A. Shavnya, S. B. Coffey, A. C. Smith and V. Mascitti, Org. Lett., 2013, 15, 6226 CrossRef CAS PubMed; (c) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2014, 53, 10204 CrossRef CAS PubMed; (d) M. W. Johnson, S. W. Bagley, N. P. Mankad, R. G. Bergman, V. Mascitti and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 4404 CrossRef CAS; (e) A. Shavnya, K. D. Hesp, V. Mascitti and A. C. Smith, Angew. Chem., Int. Ed., 2015, 54, 13571 CrossRef CAS; (f) A. S. Deeming, C. J. Russell and M. C. Willis, Angew. Chem., Int. Ed., 2016, 55, 747 CrossRef CAS; (g) V. Vedovato, E. P. A. Talbot and M. C. Willis, Org. Lett., 2018, 20, 5493 CrossRef CAS; (h) Y. Chen, P. R. D. Murray, A. T. Davies and M. C. Willis, J. Am. Chem. Soc., 2018, 140, 8781 CrossRef CAS PubMed.
- Y. An, H. Xia and J. Wu, Org. Biomol. Chem., 2016, 14, 1665 RSC.
- (a) X. Wang, L. Xue and Z. Wang, Org. Lett., 2014, 16, 4056 CrossRef CAS PubMed; (b) D. Zheng, M. Chen, L. Yao and J. Wu, Org. Chem. Front., 2016, 3, 985 RSC; (c) D. Zheng, R. Mao, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 359 RSC; (d) N. Wolff, J. Char, X. Frogneux and T. Cantat, Angew. Chem., Int. Ed., 2017, 56, 5616 CrossRef PubMed.
- (a) H. Woolven, C. González-Rodríguez, I. Marco, A. L. Thompson and M. C. Willis, Org. Lett., 2011, 13, 4876 CrossRef CAS PubMed; (b) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2013, 52, 12679 CrossRef CAS PubMed; (c) B. N. Rocke, K. B. Bahnck, M. Herr, S. Lavergne, V. Mascitti, C. Perreault, J. Polivkova and A. Shavnya, Org. Lett., 2014, 16, 154 CrossRef CAS; (d) A. S. Deeming, C. J. Russell and M. C. Willis, Angew. Chem., Int. Ed., 2015, 54, 1168 CrossRef CAS; (e) C. C. Chen and J. Waser, Org. Lett., 2015, 17, 736 CrossRef CAS PubMed; (f) D. C. Lenstra, V. Vedovato, E. F. Flegeau, J. Maydom and M. C. Willis, Org. Lett., 2016, 18, 2086 CrossRef CAS.
- For recent examples, see: (a) D. Zheng, Y. An, Z. Li and J. Wu, Angew. Chem., Int. Ed., 2014, 53, 2451 CrossRef CAS PubMed; (b) D. Zheng, J. Yu and J. Wu, Angew. Chem., Int. Ed., 2016, 55, 11925 CrossRef CAS; (c) K. Zhou, M. Chen, L. Yao and J. Wu, Org. Chem. Front., 2018, 5, 371 RSC; (d) K. Zhou, J. Zhang, L. Lai, J. Cheng, J. Sun and J. Wu, Chem. Commun., 2018, 54, 7459 RSC; (e) J. Zhang, K. Zhou and J. Wu, Org. Chem. Front., 2018, 5, 813 RSC; (f) J. Zhang, F. Zhang, L. Lai, J. Cheng, J. Sun and J. Wu, Chem. Commun., 2018, 54, 3891 RSC; (g) F.-S. He, X. Cen, S. Yang, J. Zhang, H. Xia and J. Wu, Org. Chem. Front., 2018, 5, 2437 RSC; (h) X. Wang, Y. Li, G. Qiu and J. Wu, Org. Chem. Front., 2018, 5, 2555 RSC; (i) F.-S. He, Y. Wu, J. Zhang, H. Xia and J. Wu, Org. Chem. Front., 2018, 5, 2940 RSC.
- (a) W. Li, M. Beller and X.-F. Wu, Chem. Commun., 2014, 50, 9513 RSC; (b) F. Liu, J.-Y. Wang, P. Zhou, G. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Angew. Chem., Int. Ed., 2017, 56, 15570 CrossRef CAS; (c) H. Wang, S. Sun and J. Cheng, Org. Lett., 2017, 19, 5844 CrossRef CAS PubMed; (d) Z.-J. Shen, Y.-N. Wu, C.-L. He, L. He, W.-J. Hao, A.-F. Wang, S.-J. Tu and B. Jiang, Chem. Commun., 2018, 54, 445 RSC; (e) Y. Wang, L. Deng, J. Zhou, X. Wang, H. Mei, J. Han and Y. Pan, Adv. Synth. Catal., 2018, 360, 1060 CrossRef CAS; (f) Y. Wang, L. Deng, Y. Deng and J. Han, J. Org. Chem., 2018, 83, 4674 CrossRef CAS PubMed; (g) H. Wang, B. Wang, S. Sun and J. Cheng, Org. Chem. Front., 2018, 5, 2547 RSC; (h) M. Wang, B.-C. Tang, J.-G. Wang, J.-C. Xiang, A.-Y. Guan, P.-P. Huang, W.-Y. Guo, Y.-D. Wu and A.-X. Wu, Chem. Commun., 2018, 54, 7641 RSC.
- (a) Y. Li, D. Zheng, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 574 RSC; (b) K. Zhou, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 865 RSC; (c) X. Gong, Y. Ding, X. Fan and J. Wu, Adv. Synth. Catal., 2017, 359, 2999 CrossRef CAS; (d) J. Zhang, K. Zhou, G. Qiu and J. Wu, Org. Chem. Front., 2019, 6, 36 RSC.
- (a) W. Zhang and M. Luo, Chem. Commun., 2016, 52, 298 Search PubMed; (b) N.-W. Liu, S. Liang and G. Manolikakes, Adv. Synth. Catal., 2017, 359, 1308 CrossRef CAS; (c) M. Wang, S. Chen and X. Jiang, Org. Lett., 2017, 19, 4916 CrossRef CAS PubMed; (d) D. Sun, K. Yin and R. Zhang, Chem. Commun., 2018, 54, 1335 RSC; (e) Z. Chen, N.-W. Liu, M. Bolte, H. Ren and G. Manolikakes, Green Chem., 2018, 20, 3059 RSC.
- Y. Wang, B. Du, W. Sha, J. Han and Y. Pan, Org. Chem. Front., 2017, 4, 1313 RSC.
- T. Liu, Y. Li, L. Lai, J. Cheng, J. Sun and J. Wu, Org. Lett., 2018, 20, 3605 CrossRef CAS PubMed.
- Y. Li, R. Mao and J. Wu, Org. Lett., 2017, 19, 4472 CrossRef CAS PubMed.
- For reviews: (a) D. Zheng and J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017 CrossRef; (b) G. Qiu, K. Zhou, L. Gao and J. Wu, Org. Chem. Front., 2018, 5, 691 RSC; (c) G. Liu, C. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592 RSC; (d) P. Bisseret and N. Blanchard, Org. Biomol. Chem., 2013, 11, 5393 RSC; (e) A. S. Deeming, E. J. Emmett, C. S. Richards-Taylor and M. C. Willis, Synthesis, 2014, 2701 Search PubMed; (f) E. J. Emmett and M. C. Willis, Asian J. Org. Chem., 2015, 4, 602 CrossRef CAS; (g) K. Hofman, N.-W. Liu and G. Manolikakes, Chem. – Eur. J., 2018, 24, 11852 CrossRef CAS PubMed; (h) G. Qiu, L. Lai, J. Cheng and J. Wu, Chem. Commun., 2018, 54, 10405 RSC; (i) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC; (j) S. Ye, G. Qiu and J. Wu, J. Chem. Commun., 2019, 55, 1013 RSC.
- (a) A. Shavnya, S. B. Coffey, A. C. Smith and V. Mascitti, Org. Lett., 2013, 15, 6226 CrossRef CAS PubMed; (b) Y. Li, T. Liu, G. Qiu and J. Wu, Adv. Synth. Catal., 2019 DOI:10.1002/adsc.201801445.
- (a) Z. Hang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3648 CrossRef CAS PubMed; (b) S. H. Oh, Y. R. Malpani, N. Ha, Y.-S. Jung and S. B. Han, Org. Lett., 2014, 16, 1310 CrossRef CAS PubMed; (c) Y. Li, Y. Xiang, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 1493 RSC; (d) Y. Xiang, Y. Li, Y. Kuang and J. Wu, Chem. – Eur. J., 2017, 23, 1032 CrossRef CAS PubMed; (e) Y. Li, Y. Lu, R. Mao, Z. Li and J. Wu, Org. Chem. Front., 2017, 4, 1745 RSC.
- For selected examples, see: (a) E. B. Barnett, J. Chem. Soc., 1910, 97, 63 RSC; (b) K. Nakagawa and K. Minami, Tetrahedron Lett., 1972, 13, 343 CrossRef; (c) R. C. L. Mangoni, G. Palumbo and L. Previtera, Tetrahedron Lett., 1975, 16, 1041 CrossRef; (d) R. B. dos Santos, T. J. Brocksom and U. Brocksom, Tetrahedron Lett., 1997, 38, 745 CrossRef CAS.
- (a) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed; (b) Y. Cheng, X. Gu and P. Li, Org. Lett., 2013, 15, 2664 CrossRef CAS PubMed; (c) I. Ghosh, T. Ghosh, J. I. Bardagi and B. König, Science, 2014, 346, 725 CrossRef CAS PubMed; (d) I. Ghosh, R. S. Shaikh and B. König, Angew. Chem., Int. Ed., 2017, 56, 8544 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data, and copies of 1H and 13C NMR spectra. See DOI: 10.1039/c9cc00008a |
| ‡ S. Ye and Y. Li contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |