Open Access Article
Open Access ArticleDirect grafting-from of PEDOT from a photoreactive Zr-based MOF – a novel route to electrically conductive composite materials†
Alexander
Mohmeyer
 ab,
Andreas
Schaate
ab,
Andreas
Schaate
 a,
Bastian
Hoppe
a,
Bastian
Hoppe
 a,
Hendrik A.
Schulze
a,
Hendrik A.
Schulze
 a,
Thea
Heinemeyer
a and
Peter
Behrens
a,
Thea
Heinemeyer
a and
Peter
Behrens
 *ab
*ab
aInstitute for Inorganic Chemistry, Leibniz University Hannover, Germany. E-mail: peter.behrens@acb.uni-hannover.de
bCluster of Excellence Hearing4all, Leibniz University Hannover, Germany
First published on 26th February 2019
Abstract
The postsynthetic potential of the two-dimensional metal–organic framework Zr-bzpdc-MOF which is based on the photoreactive molecule benzophenone-4,4′-dicarboxylic acid (H2bzpdc) is used here to selectively functionalize the MOF surface. We report the direct radical-induced oxidative grafting-from polymerization of the precursor EDOT on Zr-bzpdc-MOF, leading to an electrically conductive composite material and opening the road to a variety of applications.
Metal–organic frameworks (MOFs) feature an ordered structure with permanent porosity which is suitable for a variety of possible applications1 like catalysis,2,3 gas separation and storage,4,5 sensing,6,7 in biomedicine8,9 or in energy-storage devices.10 However, the direct synthesis of materials with desired yet sensitive functionalities is limited due to the synthesis conditions of MOFs. Postsynthetic modification (PSM) is a strategy to alleviate such problems. PSM summarizes different approaches like linker exchange, postsynthetic metalation or organic reactions at the linker molecules.11–15
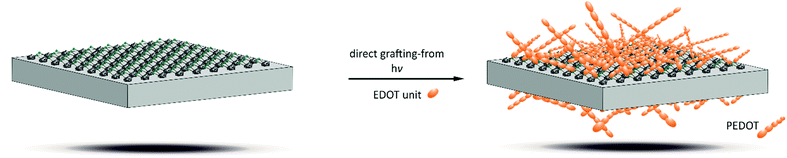
The synthetic possibilities of organic reactions at linker molecules are in principle limitless.16 Usually, grafting procedures17–20 are carried out as grafting-to reactions. Especially for the modification of crystal surfaces with polymers this approach may be problematic as the first grafted-to polymer strands may hinder further access to the reactive functionalities at the surface, resulting in a low degree of functionalization. Grafting-from reactions, where polymer chains grow in a simultaneous and parallel fashion from surface-standing polymerization-initiating entities are usually more effective. Only few grafting-from MOF polymerization reactions have been described in the literature. These usually involve additional preparatory steps as fixing polymerization-initiating groups on the surface21,22 or adding a reactive MOF shell to an innocent MOF core.22 Here, we describe grafting-from polymerizations from the surface of MOF particles directly as they are obtained after synthesis and purification. We photochemically initiated a polymerization of 3,4-ethylenedioxythiophene (EDOT) to the electrically conductive polythiophene derivative poly-ethylenedioxythiophene (PEDOT), in this way directly synthesizing an electrically conductive MOF–polymer composite (illustrated in Scheme 1).
Electrical conductivity is currently a much sought-after property in metal–organic frameworks for applications like sensing, energy storage or electronics.23–29 Also applications in coatings of neuronal electrodes could be of interest, as they would allow electrical stimulation of nerve cells/registration of neuronal signals combined with drug delivery from the pores of the MOF. However, only very few MOFs exhibit intrinsic electrical conductivity. The rare examples rely on the formation of π-stacked pathways through three-dimensional MOFs,30,31 delocalization in two-dimensional coordination polymers32–36 or via sulfur-based linker molecules37 as well as the special properties of iron-based MOFs.38–40
Alternatively, the introduction of guest molecules like tetracyanoquinododimethane,41 fullerene,42 polyaniline and polythiophene derivatives,40,43–49 as well as the formation of composites with conducting carbon modifications42,50–54 or conducting polymers55,56 can lead to conductive MOF-based materials. Generally, the introduction of guests into the pore space can compromise porosity; on the contrary, polymerization of EDOT in the pore system of MIL-101(Cr) leads to a conductive material with only slightly reduced porosity.55
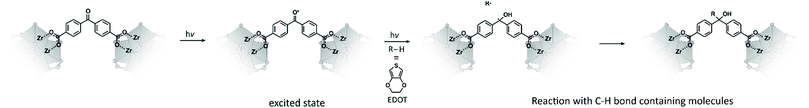
Especially for Zr-based MOFs there have been only a few studies to impart electrical conductivity on these compounds,42,46,57 although this family has been in the spotlight of research during the last years due to a controlled synthetic access using the modulation method,58,59 to their chemical and topological versatility and to their high chemical and thermal stability, as compared to other MOFs.60,61 Recently, we have described the Zr-bzpdc-MOF, which contains linkers derived from benzophenone-4,4′-dicarboxylic acid (H2bzpdc).62 This MOF has a two-dimensional structure, a moderate porosity of about 650 m2 g−1, and can be delaminated into very thin sheets. The most striking feature of the Zr-bzpdc-MOF, however, is the possibility to use the photoreactive keto groups of the linker molecules for PSM reactions. Upon excitation with light, benzophenone moieties react with practically any compound containing a C–H bond,63–65 according to Scheme 2. We have exemplarily shown that such reactions can also proceed when the bzpdc linker is part of a MOF by grafting-to reactions of the Zr-bzpdc-MOF with decane and polyethylene glycol.62 Meanwhile, we have extended these grafting-to reactions to other organic molecules and have descried that molecules with more than four carbon atoms react pre-dominantly at the outer surface of the crystals whereas smaller molecules can enter the pore system so that the reaction can take place throughout the whole crystal.66 Here, we use the ketyl radicals formed by photoexcitation of the keto groups of the Zr-bzpdc-MOF to start polymerization reactions.
The Zr-bzpdc-MOF was synthesized as described in the literature using a slightly modified procedure (Section S2.1, ESI†).62 This synthesis approach leads to rhombic shaped Zr-bzpdc-MOF crystals with only a very low amount of amorphous byproduct (Fig. S1, ESI†). The resulting colorless powder was Soxhlet-extracted with acetone for 24 h and dried under reduced pressure overnight to remove solvent molecules.
For the grafting procedure, Zr-bzpdc-MOF crystals were dispersed in neat EDOT in a quartz vessel, purged with argon for one hour and then irradiated under argon purging for a specific time with a UV LED at a wavelength of 365 nm to induce the biradical formation at the keto group of the benzophenone dicarboxylate linkers in the framework. To remove excess EDOT and non-attached oligo/polymers, the products were Soxhlet-extracted with acetone for 24 h and dried under reduced pressure (further details on the synthesis and characterization methods are given in Sections S2.1, S3.1 and S3.2 of the ESI†).
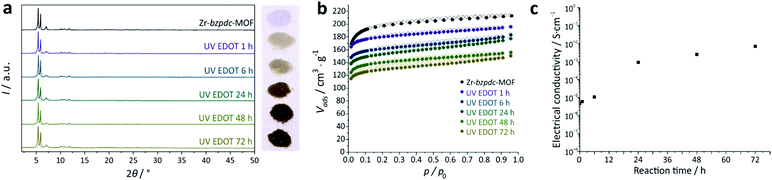
The most obvious and visible result to confirm a successful polymerization of EDOT is the strong color change of the samples after irradiation. With increasing reaction time the color of the sample changes from colorless to dark brownish (Fig. 1a). The crystallinity of the MOFs, as determined via powder X-ray diffraction, is not affected by the PSM (Fig. 1a). All reflections are present and the intensity ratios are comparable.
The investigation of the postsynthetically modified samples with energy-dispersive X-ray spectroscopy (EDX) showed a sulfur signal associated with the rhombic-shaped MOF crystals after postsynthetic modification with PEDOT (Section S3.3, ESI†). To quantify the amount of EDOT attached on the MOF surface, thermogravimetric and elemental (carbon and sulfur, C–S) analysis were performed. Thermogravimetric analysis (TGA) indicates a mass fraction of up to 2.8% of additional organic species (for the sample UV-irradiated for 72 h) (Section S3.4, ESI†). C–S analysis (Section S3.5, ESI†) gives a sulfur content of 0.76%, corresponding to a somewhat larger additional organic part of 3.4%. On the other hand, results from NMR spectroscopy (Section S3.6, ESI†) on acid-digested samples show that the vast majority of the keto groups – those located in the inner pores of the crystals – has not participated in the reaction, i.e. only the keto groups exposed on the outer surface have reacted. Using the size of the crystals and information from the crystal structure, the number of these exposed keto groups can be calculated. With the results from TGA and C–S analysis and using some assumptions we can estimate that the grafted-from PEDOT chains have lengths of at least ca. 100 EDOT units (for details of the calculations see Section S3.7, ESI†).
We postulate that the polymerization of EDOT proceeds via initiation by surface-standing ketyl radicals and subsequent oxidative polymerization (which is the common polymerization mechanism for EDOT; a corresponding reaction scheme presenting a postulated mechanism is described in Section S4 of the ESI†). Within this mechanism, the ethylene moiety of an EDOT molecule is used for its covalent attachment to the MOF surface. Subsequently, photo-excited benzophenone units of the linkers of the MOF oxidize further EDOT molecules, whereby the keto groups are reduced to alcohols. Usually, iron(III) salts or other oxidizing agents are used for the oxidative polymerization of EDOT.67 However, the photo-oxidative potential of irradiated benzophenone is well-known in literature68–70 and presents a possible route to oxidative polymerization of EDOT to PEDOT without the necessity for other oxidizing agents (which are not present in our system). The polymerization of EDOT in its neat state without prior attachment to the MOF surface cannot be ruled out, but the resulting polymer chains would not be covalently bonded to the MOF crystals and should be removed by the extended extraction performed on the samples. Therefore, we propose that the PEDOT chains are covalently bonded on the MOF surface, leading to an electrically conductive composite material.
Composites between MOFs and polymers have been described extensively.71,72 In our case, the combination of porosity and electrical conductivity is the most exciting aspect of the composite materials described here. Physisorption measurements (Fig. 1b) show a slight decrease in pore volume and surface area which cannot be explained solely by the weight increase due to the mass of the attached polymer (Table S1, ESI†). Possibly, PEDOT molecules partially block the pores resulting in a slightly decreased pore volume. The isotherms also show a slight hysteresis which becomes more pronounced with longer irradiation time. Such hysteretic sorption behavior is indicative of the presence of a soft polymer which is deformed during adsorption.73,74 It can therefore be taken as an additional indication for a layer of PEDOT molecules at the MOF surface.
The electrical conductivity of the materials was measured using a van der Pauw setup on pressed pellets. The crystallinity of the framework is not affected by the pressure used to obtain the pressed pellets (Section S3.8, ESI†). The results of the electrical conductivity measurements are shown in Fig. 1c. Surprisingly, the non-modified Zr-bzpdc-MOF shows an electrical conductivity in a range of about 10−6 S cm−1. Unexpectedly, this result places the Zr-bzpdc-MOF in the small group of intrinsically conducting MOFs. A possible explanation could be the generation of radicals at the keto groups, e.g. by former exposure to light (the actual measurement was performed in the dark under dry argon) or by the application of an electric potential during the measurements (the benzophenone moiety is known to be electrochemically reactive).75 A measurable conductivity could then result from radical delocalization within the benzophenone units76 and electron hopping between those. In fact, when we first deactivate the radical-forming benzophenone units by reacting them photochemically with ethanol (all benzophenone units within the crystals can be accessed in this way, Fig. S9, ESI†),66 there is no measurable conductivity (Section S3.8, ESI†).
The samples irradiated in EDOT show much higher conductivities than the pristine Zr-bzpdc-MOF (Table S5, ESI†). The electrical conductivity increases during the first 24 h of irradiation by more than two orders of magnitude and then saturates in the range of 10−3 S cm−1, with the highest value of 0.007 S cm−1 observed for samples which had been irradiated for 72 h (Fig. 1c). This value is in the range of semiconducting materials and fits well for various applications, e.g. sensing.
In summary, we have demonstrated the postsynthetic photochemical modification of a MOF by a grafting-from reaction, which does not need any further preparatory steps, but can be carried out directly on the MOF crystals using the surface-standing linker molecules. The resulting composite materials of the Zr-bzpdc-MOF with PEDOT show an interesting combination of moderate porosities and good electrical conductivities (Fig. S13, ESI†) so that applications as sensor material or as a biomaterial in neuronal electrodes appear feasible. This work thus reveals great potential for postsynthetic photochemical modifications reactions of the Zr-bzpdc-MOF and other MOFs containing photoreactive linkers.
This work was partially funded by the DFG EXC 1077/1 “Hearing4all”. We thank Katharina Nolte for thermogravimetric analysis and Claudia Schulze for elemental analysis. Also we thank the Institute for Organic Chemistry (Leibniz University Hannover) for performing NMR spectroscopy measurements.
Conflicts of interest
There are no conflicts to declare.Notes and references
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626–636 RSC.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- Z. Kang, L. Fan and D. Sun, J. Mater. Chem. A, 2017, 5, 10073–10091 RSC.
- B. Li, H.-M. Wen, W. Zhou and B. Chen, J. Phys. Chem. Lett., 2014, 5, 3468–3479 CrossRef CAS PubMed.
- P. Kumar, A. Deep and K.-H. Kim, Trends Anal. Chem., 2015, 73, 39–53 CrossRef CAS.
- M. Schulz, A. Gehl, J. Schlenkrich, H. A. Schulze, S. Zimmermann and A. Schaate, Angew. Chem., Int. Ed., 2018, 57, 12961–12965 CrossRef CAS PubMed.
- T. Simon-Yarza, A. Mielcarek, P. Couvreur and C. Serre, Adv. Mater., 2018, 30, 1870281 CrossRef.
- M. Giménez-Marqués, T. Hidalgo, C. Serre and P. Horcajada, Coord. Chem. Rev., 2016, 307, 342–360 CrossRef.
- V. Bon, Curr. Opin. Green Sustainable Chem., 2017, 4, 44–49 CrossRef.
- K. K. Tanabe and S. M. Cohen, Chem. Soc. Rev., 2011, 40, 498–519 RSC.
- T. Islamoglu, S. Goswami, Z. Li, A. J. Howarth, O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2017, 50, 805–813 CrossRef CAS PubMed.
- P. Roy, A. Schaate, P. Behrens and A. Godt, Chem. – Eur. J., 2012, 18, 6979–6985 CrossRef CAS PubMed.
- T. von Zons, L. Brokmann, J. Lippke, T. Preuße, M. Hülsmann, A. Schaate, P. Behrens and A. Godt, Inorg. Chem., 2018, 57, 3348–3359 CrossRef CAS PubMed.
- B. Liu, M. Ma, D. Zacher, A. Bétard, K. Yusenko, N. Metzler-Nolte, C. Wöll and R. A. Fischer, J. Am. Chem. Soc., 2011, 133, 1734–1737 CrossRef CAS PubMed.
- S. M. Cohen, J. Am. Chem. Soc., 2017, 139, 2855–2863 CrossRef CAS PubMed.
- C. Chen, J. Kim, D.-W. Park and W.-S. Ahn, Mater. Lett., 2013, 106, 344–347 CrossRef CAS.
- K. Leus, Y.-Y. Liu, M. Meledina, S. Turner, G. van Tendeloo and P. van der Voort, J. Catal., 2014, 316, 201–209 CrossRef CAS.
- N. E. Thornburg, Y. Liu, P. Li, J. T. Hupp, O. K. Farha and J. M. Notestein, Catal. Sci. Technol., 2016, 6, 6480–6484 RSC.
- X. Wang, H. Li and X.-J. Hou, J. Phys. Chem. C, 2012, 116, 19814–19821 CrossRef CAS.
- H. Liu, H. Zhu and S. Zhu, Macromol. Mater. Eng., 2015, 300, 191–197 CrossRef CAS.
- K. A. McDonald, J. I. Feldblyum, K. Koh, A. G. Wong-Foy and A. J. Matzger, Chem. Commun., 2015, 51, 11994–11996 RSC.
- L. Sun, M. G. Campbell and M. Dincă, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS PubMed.
- C.-W. Kung, Y.-S. Li, M.-H. Lee, S.-Y. Wang, W.-H. Chiang and K.-C. Ho, J. Mater. Chem. A, 2016, 4, 10673–10682 RSC.
- S. Lin, Y. Pineda-Galvan, W. A. Maza, C. C. Epley, J. Zhu, M. C. Kessinger, Y. Pushkar and A. J. Morris, ChemSusChem, 2017, 10, 514–522 CrossRef CAS PubMed.
- M. G. Campbell and M. Dincă, Sensors, 2017, 17, 1108 CrossRef PubMed.
- M. D. Allendorf, A. Schwartzberg, V. Stavila and A. A. Talin, Chem. – Eur. J., 2011, 17, 11372–11388 CrossRef CAS PubMed.
- S. K. Bhardwaj, N. Bhardwaj, R. Kaur, J. Mehta, A. L. Sharma, K.-H. Kim and A. Deep, J. Mater. Chem. A, 2018, 6, 14992–15009 RSC.
- P. Li and Bo Wang, Isr. J. Chem., 2018, 58, 1010–1018 CrossRef CAS.
- C. F. Leong, B. Chan, T. B. Faust and D. M. D'Alessandro, Chem. Sci., 2014, 5, 4724–4728 RSC.
- T. C. Narayan, T. Miyakai, S. Seki and M. Dincă, J. Am. Chem. Soc., 2012, 134, 12932–12935 CrossRef CAS PubMed.
- M. G. Campbell, S. F. Liu, T. M. Swager and M. Dincă, J. Am. Chem. Soc., 2015, 137, 13780–13783 CrossRef CAS PubMed.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. Dincă, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 CrossRef CAS PubMed.
- M. Hmadeh, Z. Lu, Z. Liu, F. Gándara, H. Furukawa, S. Wan, V. Augustyn, R. Chang, L. Liao, F. Zhou, E. Perre, V. Ozolins, K. Suenaga, X. Duan, B. Dunn, Y. Yamamto, O. Terasaki and O. M. Yaghi, Chem. Mater., 2012, 24, 3511–3513 CrossRef CAS.
- X. Huang, P. Sheng, Z. Tu, F. Zhang, J. Wang, H. Geng, Y. Zou, C.-A. Di, Y. Yi, Y. Sun, W. Xu and D. Zhu, Nat. Commun., 2015, 6, 7408 CrossRef CAS PubMed.
- B. Hoppe, K. D. J. Hindricks, D. P. Warwas, H. A. Schulze, A. Mohmeyer, T. J. Pinkvos, S. Zailskas, M. R. Krey, C. Belke, S. König, M. Fröba, R. J. Haug and P. Behrens, CrystEngComm, 2018, 20, 6458–6471 RSC.
- F. Li, X. Zhang, X. Liu and M. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 15012–15020 CrossRef CAS PubMed.
- L. Sun, C. H. Hendon, S. S. Park, Y. Tulchinsky, R. Wan, F. Wang, A. Walsh and M. Dincă, Chem. Sci., 2017, 8, 4450–4457 RSC.
- J. G. Park, M. L. Aubrey, J. Oktawiec, K. Chakarawet, L. E. Darago, F. Grandjean, G. J. Long and Jeffrey R. Long, J. Am. Chem. Soc., 2018, 140, 8526–8534 CrossRef CAS PubMed.
- M. L. Aubrey, B. M. Wiers, S. C. Andrews, T. Sakurai, S. E. Reyes-Lillo, S. M. Hamed, C.-J. Yu, L. E. Darago, J. A. Mason, J.-O. Baeg, F. Grandjean, G. J. Long, S. Seki, J. B. Neaton, P. Yang and J. R. Long, Nat. Mater., 2018, 17, 625–632 CrossRef CAS PubMed.
- A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly, H. P. Yoon, F. Léonard and M. D. Allendorf, Science, 2014, 343, 66–69 CrossRef CAS PubMed.
- S. Goswami, D. Ray, K.-I. Otake, C.-W. Kung, S. J. Garibay, T. Islamoglu, A. Atilgan, Y. Cui, C. J. Cramer, O. K. Farha and J. T. Hupp, Chem. Sci., 2018, 9, 4477–4482 RSC.
- C. Lu, T. Ben, S. Xu and S. Qiu, Angew. Chem., Int. Ed., 2014, 53, 6454–6458 CrossRef CAS PubMed.
- H. Shiozawa, B. C. Bayer, H. Peterlik, J. C. Meyer, W. Lang and T. Pichler, Sci. Rep., 2017, 7, 2439 CrossRef PubMed.
- Y. Wang, L. Wang, W. Huang, T. Zhang, X. Hu, J. A. Perman and S. Ma, J. Mater. Chem. A, 2017, 5, 8385–8393 RSC.
- T. Wang, M. Farajollahi, S. Henke, T. Zhu, S. R. Bajpe, S. Sun, J. S. Barnard, J. S. Lee, J. D. W. Madden, A. K. Cheetham and S. K. Smoukov, Mater. Horiz., 2017, 4, 64–71 RSC.
- D. Fu, H. Zhou, X.-M. Zhang, G. Han, Y. Chang and H. Li, ChemistrySelect, 2016, 1, 285–289 CrossRef CAS.
- T.-Y. Huang, C.-W. Kung, Y.-T. Liao, S.-Y. Kao, M. Cheng, T.-H. Chang, J. Henzie, H. R. Alamri, Z. A. Alothman, Y. Yamauchi, K.-C. Ho and K. C.-W. Wu, Adv. Sci., 2017, 4, 1700261 CrossRef PubMed.
- C.-W. Kung, K. Otake, C. T. Buru, S. Goswami, Y. Cui, J. T. Hupp, A. M. Spokoyny and O. K. Farha, J. Am. Chem. Soc., 2018, 140, 3871–3875 CrossRef CAS PubMed.
- I. Ahmed and S. H. Jhung, Mater. Today, 2014, 17, 136–146 CrossRef CAS.
- O. Fleker, A. Borenstein, R. Lavi, L. Benisvy, S. Ruthstein and D. Aurbach, Langmuir, 2016, 32, 4935–4944 CrossRef CAS PubMed.
- P. Freund, I. Senkovska and S. Kaskel, ACS Appl. Mater. Interfaces, 2017, 9, 43782–43789 CrossRef CAS PubMed.
- Y. Mao, G. Li, Y. Guo, Z. Li, C. Liang, X. Peng and Z. Lin, Nat. Commun., 2017, 8, 14628 CrossRef PubMed.
- X. Xu, W. Shi, P. Li, S. Ye, C. Ye, H. Ye, T. Lu, A. Zheng, J. Zhu, L. Xu, M. Zhong and X. Cao, Chem. Mater., 2017, 29, 6058–6065 CrossRef CAS.
- B. Le Ouay, M. Boudot, T. Kitao, T. Yanagida, S. Kitagawa and T. Uemura, J. Am. Chem. Soc., 2016, 138, 10088–10091 CrossRef CAS PubMed.
- R. Haldar, B. Sen, S. Hurrle, T. Kitao, R. Sankhla, B. Kühl, A. Welle, S. Heissler, G. Brenner-Weiß, P. Thissen, T. Uemura, H. Gliemann, C. Barner-Kowollik and C. Wöll, Eur. Polym. J., 2018, 109, 162–168 CrossRef CAS.
- T. C. Wang, I. Hod, C. O. Audu, N. A. Vermeulen, S. T. Nguyen, O. K. Farha and J. T. Hupp, ACS Appl. Mater. Interfaces, 2017, 9, 12584–12591 CrossRef CAS PubMed.
- G. Zahn, H. A. Schulze, J. Lippke, S. König, U. Sazama, M. Fröba and P. Behrens, Microporous Mesoporous Mater., 2015, 203, 186–194 CrossRef CAS.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem. – Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- J. E. Mondloch, M. J. Katz, N. Planas, D. Semrouni, L. Gagliardi, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 8944–8946 RSC.
- Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- A. Mohmeyer, A. Schaate, B. Brechtken, J. C. Rode, D. P. Warwas, G. Zahn, R. J. Haug and P. Behrens, Chem. – Eur. J., 2018, 24, 12848–12855 CrossRef CAS PubMed.
- D. Karaca Balta, Ö. Karahan, D. Avci and N. Arsu, Prog. Org. Coat., 2015, 78, 200–207 CrossRef CAS.
- M. A. Winnik and U. Maharaj, Macromolecules, 1979, 12, 902–905 CrossRef CAS.
- O. Prucker, C. A. Naumann, J. Rühe, W. Knoll and C. W. Frank, J. Am. Chem. Soc., 1999, 121, 8766–8770 CrossRef CAS.
- A. Mohmeyer, M. Schäfer, A. Schaate, S. Locmelis and P. Behrens, unpublished results.
- T. Horii, H. Hikawa, M. Katsunuma and H. Okuzaki, Polymer, 2018, 140, 33–38 CrossRef CAS.
- N. Filipescu and F. L. Minn, J. Am. Chem. Soc., 1968, 90, 1544–1547 CrossRef CAS.
- B. Qu, Y. Xu, L. Ding and B. Rånby, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 999–1005 CrossRef CAS.
- A. Demeter, K. Horváth, K. Böőr, L. Molnár, T. Soós and G. Lendvay, J. Phys. Chem. A, 2013, 117, 10196–10210 CrossRef CAS PubMed.
- T. Kitao, Y. Zhang, S. Kitagawa, B. Wang and T. Uemura, Chem. Soc. Rev., 2017, 46, 3108–3133 RSC.
- S. Mochizuki, T. Kitao and T. Uemura, Chem. Commun., 2018, 54, 11843–11856 RSC.
- Y. Tsujita, Prog. Polym. Sci., 2003, 28, 1377–1401 CrossRef CAS.
- J. Weber, M. Antonietti and A. Thomas, Macromolecules, 2008, 41, 2880–2885 CrossRef CAS.
- N. G. Tsierkezos and U. Ritter, Phys. Chem. Liq., 2011, 49, 729–742 CrossRef CAS.
- Y. Du, J. Xue, M. Li and D. L. Phillips, J. Phys. Chem. A, 2009, 113, 3344–3352 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation details. See DOI: 10.1039/c8cc10298h |
| This journal is © The Royal Society of Chemistry 2019 |