Open Access Article
Open Access Article(PhC(NtBu)2Al)2(SiH2)4 six-membered heterocycle: comparable in structure to cyclohexane†
Jiancheng
Li
ab,
Mingdong
Zhong
b,
Helena
Keil
b,
Hongping
Zhu
 *a,
Regine
Herbst-Irmer
*a,
Regine
Herbst-Irmer
 b,
Dietmar
Stalke
b,
Dietmar
Stalke
 *b,
Sriman
De
*b,
Sriman
De
 c,
Debasis
Koley
c,
Debasis
Koley
 *c and
Herbert W.
Roesky
*c and
Herbert W.
Roesky
 *b
*b
aState Key Laboratory of Physical Chemistry of Solid Surface, National Engineering Laboratory for Green Chemical Productions of Alcohols-Ethers-Esters College of Chemistry and Chemical Engineering Xiamen University, Xiamen 361005, China. E-mail: hpzhu@xmu.edu.cn
bInstitut für Anorganische Chemie, Universität Göttingen, Tammannstraße 4, 37077 Göttingen, Germany. E-mail: hroesky@gwdg.de; dstalke@chemie.uni-goettingen.de
cDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur-741 246, India. E-mail: koley@iiserkol.ac.in
First published on 25th January 2019
Abstract
A silicon–aluminum heterocycle LAl(SiH2SiH2)2AlL (L = PhC(NtBu)2) (1) was prepared. Compound 1 exhibits a unique (N2Al)2(SiH2)4 centrosymmetric six-membered ring structure with a chair conformation, which is comparable with that of cyclohexane. Furthermore, two intermediate analogues, silylene–alane adduct LSi(AlMe3)–Si(AlMe3)L (2) and silylene–alane oxidative product [LAlHSiH2Mes]2 (3) were obtained. Compound 3 has an interesting arrangement of an Al–H and an SiH2 unit, which are in close vicinity to each other. 3 might be important to function as a catalyst, due to the already activated bridging Al–H bonds.
Aluminum is the most abundant metal and silicon is the second most abundant element in the earth's crust.1 Due to their convenient availability and broad use in industry,2 the research of aluminum and silicon chemistry is very important. In recent decades, our group and others have focused on low-valent silicon and aluminum chemistry and many representative compounds, such as LSiR (L = PhC(NtBu)2),3 LSi–SiL (L = PhC(NtBu)2),4 N-heterocyclic silylenes (NHSi)5 and [Cp*Al]4,6 have been prepared using alkaline metals or non-metals as reducing agents. Recently, cyclic silicon and cyclic aluminum compounds have attracted much attention because of their unique bonding nature and reactivities.7 For example, three-silicon membered cyclic compounds R4Si3 (R = SitBu2Me or 2, 4, 6-iPr3C6H2) were reported by Sekiguchi8 and Scheschkewitz.9 Tamao10 and Driess11 reported a neutral tetrasilacyclobutadiene (EMind)4Si4 and a tetrasilacyclobutadiene dication [L2Si2Si2(:Si(Cl)L)]2+ (L = PhC(NtBu)2) both containing a central Si4 ring. The most extensively studied cyclic silicon compounds are cyclohexasilanes, Si6X12 (X = H,12 Me,13 Ph,14 halides15), which were prepared by the reduction of silicon halides or by using a silicon anion. It is worth mentioning that Scheschkewitz et al. reported a silicon analogue of benzene R6Si6 (R = 2,4,6-iPr3C6H2), which is aromatic.16 Subsequently, a large amount of research has been dedicated to studying hexasilabenzenes and their isomers.17 Compared to silicon, however, cyclic aluminum compounds are more likely to form aluminum clusters.6,18 Nevertheless, Power19 reported compounds with a central three-membered Al3-ring of [Ar*3Al3]2+ (Ar* = 2,6-Tip2C6H3, Tip = 2,4,6-iPr3C6H2) by using bulky substituents.
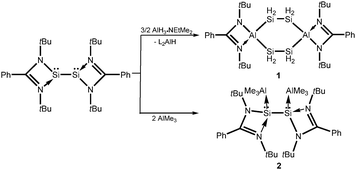
Despite the numerous reports on cyclic silicon and aluminum compounds, to the best of our knowledge, molecular cyclic compounds containing silicon and aluminum are not known. However, aluminum-doped silicon clusters contain these combinations, and their structures were only studied by theoretical calculations.20 The difficulty in preparing silicon–aluminum heterocycles is probably due to the metathesis of the silicon anion with aluminum halides. Generally, this could realize the formation of a compound with acyclic Si–Al bonds.21 Furthermore, compounds containing the Al–SiH2R unit are rarely reported, probably due to the limited utility of the RH2Si− anion. So far only Nikonov et al. reported on the oxidative addition of the Si–H bond of PhSiH3 to the Al(I) center of NacnacAl(:), resulting in the formation of NacnacAlHSiH2Ph.22 However, this compound does not show any hydrogen bridging character. Inspired by the high reactivity of low-valent silicon explored by our group,3,4 we recently were interested in the reactions of amidinate supported low-valent silicon with aluminum compounds.‡ Herein, we report two unexpected results of the synthesis of Al2(SiH2)4 six-membered rings, where two silicon atoms of cyclohexasilane are replaced by two aluminum atoms to yield LAl(SiH2SiH2)2AlL (1) and silylene–alane oxidative product [LAlHSiH2Mes]2 (3). The latter and the silylene–alane adduct LSi(AlMe3)–Si(AlMe3)L (2) were prepared to get a better insight into the formation of compound 1. Compounds 2 and 3 are important species to explain the formation of 1.
We initially attempted the reaction of disilylene LSi–SiL (L = PhC(NtBu)2) with AlH3·NEtMe2 in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in toluene in the temperature range from −78 °C to room temperature (Scheme 1). Colorless crystals were obtained of composition L2AlH,23 which was confirmed by NMR and mass spectrometry. However, attempts to separate a second silicon compound failed. We then changed the ratio of the precursors to 2
2 in toluene in the temperature range from −78 °C to room temperature (Scheme 1). Colorless crystals were obtained of composition L2AlH,23 which was confirmed by NMR and mass spectrometry. However, attempts to separate a second silicon compound failed. We then changed the ratio of the precursors to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and a similar color change of the solution from red to colorless was observed. After separation of L2AlH, we unexpectly obtained LAl(SiH2SiH2)2AlL (1) as a crystalline solid in low yield (18%) (Scheme 1). Similar to reported results of the reaction between silylene and borane,24 we propose that on the basis of the ratio of the precursors and the final results, the disilanylaluminum intermediate LAlHSiH2SiH2AlHL is unstable. It presumably reacts further with another equivalent of disilylene LSi–SiL in the presence of an equivalent amount of alane AlH3·NEtMe2. Unfortunately, the attempt to characterize the intermediates was unsuccessful. However, for comparison we treated LSi–SiL with AlMe3 and isolated only product 2 as a colorless crystalline solid (Scheme 1). Compound 2 is a Lewis acid–base adduct, with the Si atom acting as a two electron donor. Note, AlMe3 undergoes oxidative addition at the Si(II) atom of N-heterocyclic silylene LSi: (L = (ArN)C(CH2)CHC(Me)(NAr), Ar = 2,6-iPr2C6H3).25 The formation of compound 2 suggests that compound 1 also proceeds under adduct formation of LSi–SiL with two AlH3 followed by the insertion of the Si atom into the Al–H bonds.
3 and a similar color change of the solution from red to colorless was observed. After separation of L2AlH, we unexpectly obtained LAl(SiH2SiH2)2AlL (1) as a crystalline solid in low yield (18%) (Scheme 1). Similar to reported results of the reaction between silylene and borane,24 we propose that on the basis of the ratio of the precursors and the final results, the disilanylaluminum intermediate LAlHSiH2SiH2AlHL is unstable. It presumably reacts further with another equivalent of disilylene LSi–SiL in the presence of an equivalent amount of alane AlH3·NEtMe2. Unfortunately, the attempt to characterize the intermediates was unsuccessful. However, for comparison we treated LSi–SiL with AlMe3 and isolated only product 2 as a colorless crystalline solid (Scheme 1). Compound 2 is a Lewis acid–base adduct, with the Si atom acting as a two electron donor. Note, AlMe3 undergoes oxidative addition at the Si(II) atom of N-heterocyclic silylene LSi: (L = (ArN)C(CH2)CHC(Me)(NAr), Ar = 2,6-iPr2C6H3).25 The formation of compound 2 suggests that compound 1 also proceeds under adduct formation of LSi–SiL with two AlH3 followed by the insertion of the Si atom into the Al–H bonds.
Compounds 1 and 2 are air and moisture sensitive. Under N2 atmosphere, they are both stable for more than one month in the solid state. However, compound 1 in solution decomposed into an unidentified mixture within a few days at room temperature. For compound 1, the 29Si INEPT spectrum displays a broad resonance at −128.9 ppm, which is highly upfield shifted when compared with that of compound LSi–SiL (76.3 ppm).4 However, in the 29Si NMR spectrum of compound 2, the Si resonance is at +56.9 ppm, indicating remaining silylene character. The Si–H hydrides of compound 2 give rise to the 1H NMR resonance at 3.36 ppm, which is upfield-shifted when compared with that of compound 3 (4.54 ppm, see below) and NacnacAlHSiH2Ph (3.87 ppm).22 The IR spectrum of compound 2 displays a broad band at 2074 cm−1, attributed to Si–H bond stretching. No resonance for the Al–H bond is observed. In the 1H NMR spectrum of compound 2, the resonances for tBu groups on the amidinate ligands and methyl groups on aluminum were observed at 1.21 ppm and −0.02 ppm, respectively.
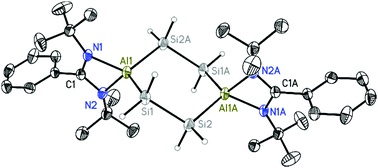
Single-crystal X-ray structural analysis of compound 1 clearly revealed a centrosymmetric six-membered heterocycle with two Al atoms at the 1,4-positions which are linked by two –SiH2–SiH2– units (Fig. 1). The Al2Si4 heterocycle adopts a chair conformation, typical of cyclohexane congeners where the six methylene groups are replaced by two N2Al units and four SiH2 moieties. These four silicon atoms are in the same plane while one Al atom is located above and one below that Si4 plane. The dihedral angle between the planes defined by Si4 and Al1Si1Si2A is 50.03(4)°, while the distance between the Al atom and the Si4 plane is 1.0113(10) Å. The bond lengths of Al–Si (2.4473(9) and 2.4545(9) Å) in 1 are comparable with those in NacnacAlHSiH2Ph (2.4522(8) Å),22 NacnacAlHSiHMePh (2.4548(7) Å)22 and LSiHAlH2(NMe3) (L = (ArN)C(CH2)CHC(Me)(NAr), Ar = 2,6-iPr2C6H3) (2.487(1) Å).25 The two SiH2–SiH2 bond lengths (2.3413(9) Å) are crystallographically equivalent and comparable with the corresponding distances in the (tBuN)2(SiH2)4 heterocycle (2.337(1) and 2.334(1) Å).26 The Si–H distances (1.440(17) to 1.448(18) Å) fall in the common range of Si–H bonds. Both Al atoms in compound 1 show distorted tetrahedral coordination environments, with both Al atoms at the spiro centers of six-membered (Al2Si4) and four-membered (CN2Al) rings. The ligand bite angle N1–Al1–N2 (69.47(7)°) is rather sharp, whereas the Si1–Al1–Si2A angle (114.84(3)°) is rather wide. However, the Si-centered bond angle in Al2Si4 (Al1–Si1–Si2 (107.57(3)°)) is close to the ideal tetrahedral angle of 109.47°. Compound 2 was also characterized by X-ray crystallography (see ESI†). The molecular structure of 2 shows that each silicon atom coordinates to one aluminum atom. The torsion angle Al–Si–Si–Al is −68.57(4)°. To the best of our knowledge, compound 2 represents a rare example of silylene−alane adducts, and the only other two examples are [PhC(NiPr)2]2Si → AlPh3 and [C(NiPr)3]2Si → AlPh3.27 The Si → Al dative bond lengths (2.5921(7) and 2.5799(8) Å) in compound 2 are much longer than the Si–Al σ bonds in compound 1 (2.4473(9) and 2.4545(9) Å). However, they are comparable with those in [PhC(NiPr)2]2Si → AlPh3 (2.5293(14) Å) and [C(NiPr)3]2Si → AlPh3 (2.5544(17) Å). The Si1–Si2 bond (2.3937(7) Å) is comparable with those in LSi–SiL (2.413(2) Å) and LSi(→ M)–Si(→ M)L adducts (2.376(5) Å for M = Ir and 2.388(2) Å for M = Rh).28
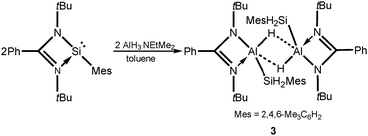
In order to get the analogues of intermediate C (Scheme 3) in the formation of compound 1, we turned to the reaction of amidinate mesitylsilylene LSiMes29 (Mes = 2,4,6-Me3C6H2) with alane AlH3·NEtMe2 in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
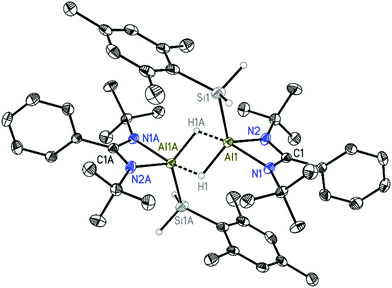
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was accomplished in toluene in the temperature range from −78 °C to room temperature (Scheme 2). After the removal of all volatiles and treating the residue with n-hexane, a white solid of [LAlHSiH2Mes]2 (3) was collected by filtration in 76% yield. Compound 3 was characterized by 1H, 13C, and 29Si NMR spectroscopy and single crystal X-ray structure analysis. The 29Si NMR spectrum of 3 shows a broad resonance at −91.7 ppm that is shifted upfield with respect to that of LSiMes (24.0 ppm)29 and NacnacAlHSiH2Ph (−74.3 ppm),22 but downfield compared with that of compound 1 (−128.9 ppm). In the 1H NMR spectrum, the broad resonance at 5.26 ppm for two hydrogens is attributed to the aluminum hydride Al–H, and the singlet at 4.54 ppm for four hydrogen atoms is attributed to silane hydrides SiH2. The existence of aluminum hydrides and silicon hydrides is also proved by the observation of broad bands at 1645 cm−1 and 2095 cm−1 in the IR spectra, respectively. The resonance for the tBu group of the amidinate ligand appears at 0.93 ppm and the characteristic resonances for the mesityl group were observed at 2.18, 2.70, and 6.88 ppm, respectively. The structure of 3 was further confirmed in a single crystal X-ray diffraction study (Fig. 2). It unambiguously displays that it dimerizes by two bridging hydrogen atoms from each aluminum, and the bidentate ligand L (L = PhC(NtBu)2) coordinates to the Al atom together with the SiH2Mes group. The five-coordinated Al atom adopts a distorted square-based pyramidal rather than a trigonal bipyramidal geometry. The Si1–Al1 bond length of 2.4602(8) Å is slightly longer than that in compound 1 (2.4473(9) and 2.4545(9) Å). The two Al–H bond distances are 1.653(16) Å and 1.796(17) Å, with one slightly longer and the other one slightly shorter than those of corresponding bonds in similar aluminum hydride dimers (LAlH2)2 (L = RC(NAr)2, R = N(iPr)2, Ar = 2,6-iPr2C6H3) (1.60(2) Å and 1.81(2) Å)30 and 2-aminophenylaluminum dihydride [(2-tmp-C6H4)AlH2]2 (1.585(16) Å and 1.896(16) Å),31 indicating in 3 a strong tendency of forming an equal Al–H bond length within a stable dimer.
1, which was accomplished in toluene in the temperature range from −78 °C to room temperature (Scheme 2). After the removal of all volatiles and treating the residue with n-hexane, a white solid of [LAlHSiH2Mes]2 (3) was collected by filtration in 76% yield. Compound 3 was characterized by 1H, 13C, and 29Si NMR spectroscopy and single crystal X-ray structure analysis. The 29Si NMR spectrum of 3 shows a broad resonance at −91.7 ppm that is shifted upfield with respect to that of LSiMes (24.0 ppm)29 and NacnacAlHSiH2Ph (−74.3 ppm),22 but downfield compared with that of compound 1 (−128.9 ppm). In the 1H NMR spectrum, the broad resonance at 5.26 ppm for two hydrogens is attributed to the aluminum hydride Al–H, and the singlet at 4.54 ppm for four hydrogen atoms is attributed to silane hydrides SiH2. The existence of aluminum hydrides and silicon hydrides is also proved by the observation of broad bands at 1645 cm−1 and 2095 cm−1 in the IR spectra, respectively. The resonance for the tBu group of the amidinate ligand appears at 0.93 ppm and the characteristic resonances for the mesityl group were observed at 2.18, 2.70, and 6.88 ppm, respectively. The structure of 3 was further confirmed in a single crystal X-ray diffraction study (Fig. 2). It unambiguously displays that it dimerizes by two bridging hydrogen atoms from each aluminum, and the bidentate ligand L (L = PhC(NtBu)2) coordinates to the Al atom together with the SiH2Mes group. The five-coordinated Al atom adopts a distorted square-based pyramidal rather than a trigonal bipyramidal geometry. The Si1–Al1 bond length of 2.4602(8) Å is slightly longer than that in compound 1 (2.4473(9) and 2.4545(9) Å). The two Al–H bond distances are 1.653(16) Å and 1.796(17) Å, with one slightly longer and the other one slightly shorter than those of corresponding bonds in similar aluminum hydride dimers (LAlH2)2 (L = RC(NAr)2, R = N(iPr)2, Ar = 2,6-iPr2C6H3) (1.60(2) Å and 1.81(2) Å)30 and 2-aminophenylaluminum dihydride [(2-tmp-C6H4)AlH2]2 (1.585(16) Å and 1.896(16) Å),31 indicating in 3 a strong tendency of forming an equal Al–H bond length within a stable dimer.
Finally, DFT calculations (see computational details, ESI†) are performed to support the mechanism for the generation of silicon–aluminum heterocycle 1. The reaction energy (I + AlH3 → 1) is highly exergonic (ΔGSL = −188.5 kcal mol−1) indicating a facile, thermodynamically favorable product formation (Scheme 3). The reaction begins with two successive oxidative additions of AlH3 at two different Si centers leading to the formation of intermediate IC. Thereafter, subsequent migrations of the hydride from Al to Si centers allow the formation of intermediate C (Fig. S3.1 and S3.2, ESI†). Finally, the addition of AlH3 and I with intermediate C furnished 1 by releasing one molecule of L2AlH. This step is highly exergonic (ΔGSL = −91.7 kcal mol−1) with respect to intermediate C. The first hydride transfer step (IC → ID; Δ‡GSL = 32.6 kcal mol−1; Fig. S3.1, ESI†)24,32 was calculated to be the rate determining step for the overall transformation, which justifies the formation of 1 with a very low yield.
 | ||
| Scheme 3 Relative Gibbs free energy (in kcal mol−1) for the intermediates involved in the formation of 1. Values in parentheses are energy values (ΔGSL; in kcal mol−1) relative to the starting material (I + AlH3). Values above the arrows are the activation barriers between the two corresponding intermediates. For further details see the ESI.† | ||
In conclusion, we report a new method for preparing a silicon–aluminum heterocycle by insertion reaction of a low valent silicon atom supported by an amidinate ligand into the Al–H bond of an alane, which is followed by a rearrangement of the amidinate ligand from Si to Al and subsequent migration of hydride from aluminum to silicon. LAl(SiH2SiH2)2AlL (1) exhibits a unique centrosymmetric six-membered ring with chair conformation, which is reminiscent of cyclohexane. 1 contains 4 silicon and two aluminum atoms, and exhibits a new six-membered ring system. In addition, LSi(AlMe3)–Si(AlMe3)L (2) and [LAlHSiH2Mes]2 (3) were prepared, which could be viewed as two intermediate analogues, indicating that the formation of compound 2 involves a silylene−alane adduct, a silylene–alane oxidative addition and finally rearrangement of the ligand along with migration of a hydride. The formation of 1 was also explored employing DFT calculations. Compound 3 is prone to generating aluminum cations that could function as catalysts.
H. W. R. thanks the Deutsche Forschungsgemeinschaft for financial support RO224/68-1. J. Li thanks the China Scholarship Council (CSC) for the fellowship (201706310031). D. S. thanks the Danish National Research Foundation (DNRF93) funded Centre for Materials Crystallography (CMC) for partial support. S. D. thanks UGC for an SRF fellowship, and D. K. acknowledges IISER Kolkata for the financial support. This study is dedicated to Professor Hubert Schmidbaur.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. R. Taylor, Geochim. Cosmochim. Acta, 1964, 28, 1273–1285 CrossRef CAS.
- (a) G. E. Totten and D. S. Mackenzie, Handbook of Aluminum, CRC Press, New York, 2003 Search PubMed; (b) J. Boor, Zieglar–Natta catalysts and polymerization, Academic Press, New York, 1979 Search PubMed; (c) G. A. Olah, Friedel-Crafts and related reactions, Wiley, New York, 1963 Search PubMed; (d) F. Peng, Y. Su, Y. Zhong, C. Fan, S.-T. Lee and Y. He, Acc. Chem. Res., 2014, 47, 612–623 CrossRef CAS PubMed.
- (a) S. S. Sen, H. W. Roesky, D. Stern, J. Henn and D. Stalke, J. Am. Chem. Soc., 2010, 132, 1123–1126 CrossRef CAS PubMed; (b) R. Azhakar, R. S. Ghadwal, H. W. Roesky, H. Wolf and D. Stalke, Organometallics, 2012, 31, 4588–4592 CrossRef CAS; (c) R. Azhakar, R. S. Ghadwal, H. W. Roesky, H. Wolf and D. Stalke, Chem. Commun., 2012, 48, 4561–4563 RSC.
- (a) S. S. Sen, A. Jana, H. W. Roesky and C. Schulzke, Angew. Chem., Int. Ed., 2009, 48, 8536–8538 CrossRef CAS PubMed; (b) S. S. Sen, S. Khan, S. Nagendran and H. W. Roesky, Acc. Chem. Res., 2012, 45, 578–587 CrossRef CAS PubMed.
- (a) M. Denk, R. Lennon, R. Hayashi, R. West, A. V. Belyakov, H. P. Verne, A. Haaland, M. Wagner and N. Metzler, J. Am. Chem. Soc., 1994, 116, 2691–2692 CrossRef CAS; (b) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354–396 CrossRef CAS PubMed.
- (a) C. Dohmeier, C. Robl, M. Tacke and H. Schnockel, Angew. Chem., Int. Ed. Engl., 1991, 30, 564–565 CrossRef; (b) S. Schulz, H. W. Roesky, H. J. Koch, G. M. Sheldrick, D. Stalke and A. Kuhn, Angew. Chem., Int. Ed. Engl., 1993, 32, 1729–1731 CrossRef.
- (a) A. Rammo and D. Scheschkewitz, Chem. – Eur. J., 2018, 24, 6866–6885 CrossRef CAS PubMed; (b) P. Bag, C. Weetman and S. Inoue, Angew. Chem., Int. Ed., 2018, 57, 14394–14413 CrossRef CAS PubMed.
- M. Ichinohe, T. Matsuno and A. Sekiguchi, Angew. Chem., Int. Ed., 1999, 38, 2194–2196 CrossRef CAS PubMed.
- K. Leszczyńska, K. Abersfelder, A. Mix, B. Neumann, H.-G. Stammler, M. J. Cowley, P. Jutzi and D. Scheschkewitz, Angew. Chem., Int. Ed., 2012, 51, 6785–6788 CrossRef PubMed.
- K. Suzuki, T. Matsuo, D. Hashizume, H. Fueno, K. Tanaka and K. Tamao, Science, 2011, 331, 1306–1309 CrossRef CAS PubMed.
- S. Inoue, J. D. Epping, E. Irran and M. Driess, J. Am. Chem. Soc., 2011, 133, 8514–8517 CrossRef CAS PubMed.
- E. Hengge and D. Kovar, Angew. Chem., Int. Ed. Engl., 1977, 16, 403 CrossRef.
- (a) C. A. Burkhard, J. Am. Chem. Soc., 1949, 71, 963–964 CrossRef CAS; (b) H. Gilman and R. A. Tomasi, J. Org. Chem., 1963, 28, 1651–1653 CrossRef CAS.
- (a) H. J. S. Winkler, A. W. P. Jarvie, D. J. Peterson and H. Gilman, J. Am. Chem. Soc., 1961, 83, 4089–4093 CrossRef CAS; (b) H. Gilman, D. J. Peterson, A. W. P. Jarvie and H. J. S. Winkler, Tetrahedron Lett., 1960, 23, 5–7 CrossRef.
- X. Dai, D. L. Schulz, C. W. Braun, A. Ugrinov and P. Boudjouk, Organometallics, 2010, 29, 2203–2205 CrossRef CAS.
- (a) K. Abersfelder, A. J. P. White, H. S. Rzepa and D. Scheschkewitz, Science, 2010, 327, 564–566 CrossRef CAS PubMed; (b) K. Abersfelder, A. J. P. White, R. J. F. Berger, H. S. Rzepa and D. Scheschkewitz, Angew. Chem., Int. Ed., 2011, 50, 7936–7939 CrossRef CAS PubMed; (c) D. Kratzert, D. Leusser, J. J. Holstein, B. Dittrich, K. Abersfelder, D. Scheschkewitz and D. Stalke, Angew. Chem., Int. Ed., 2013, 52, 4478–4482 CrossRef CAS PubMed.
- (a) K. Abersfelder, A. Russell, H. S. Rzepa, A. J. P. White, P. R. Haycock and D. Scheschkewitz, J. Am. Chem. Soc., 2012, 134, 16008–16016 CrossRef CAS PubMed; (b) A. Tsurusaki, C. Iizuka, K. Otsuka and S. Kyushin, J. Am. Chem. Soc., 2013, 135, 16340–16343 CrossRef CAS PubMed; (c) T. Szilvási and T. Veszprémi, Organometallics, 2012, 31, 3207–3212 CrossRef; (d) Z. Benedek, T. Szilvási and T. Veszprémi, Dalton Trans., 2014, 43, 1184–1190 RSC.
- (a) V. W. Hiller, K.-W. Klinkhammer, W. Uhl and J. Wagner, Angew. Chem., Int. Ed. Engl., 1991, 30, 179–180 CrossRef; (b) V. U. Schneider, R. Ahlrichs, H. Horn and A. Schäfer, Angew. Chem., Int. Ed. Engl., 1992, 31, 353–355 CrossRef.
- R. J. Wright, M. Brynda and P. P. Power, Angew. Chem., Int. Ed., 2006, 45, 5953–5956 CrossRef CAS PubMed.
- (a) M. Akutsu, K. Koyasu, J. Atobe, K. Miyajima, M. Mitsui, H. Tsunoyama and A. Nakajima, Phys. Chem. Chem. Phys., 2017, 19, 20401–20411 RSC; (b) O. P. Charkin and N. M. Klimenko, Russ. J. Inorg. Chem., 2016, 61, 594–603 CrossRef CAS; (c) N. M. Tam, T. B. Tai, V. T. Ngan and M. T. Nguyen, J. Phys. Chem. A, 2013, 117, 6867–6882 CrossRef PubMed; (d) M. Su, N. Du and H. Chen, J. Cluster Sci., 2018, 29, 141–150 CrossRef CAS.
- (a) P. Bag, A. Porzelt, P. J. Altmann and S. Inoue, J. Am. Chem. Soc., 2017, 139, 14384–14387 CrossRef CAS PubMed; (b) M. Nakamoto, T. Yamasaki and A. Sekiguchi, J. Am. Chem. Soc., 2005, 127, 6954–6955 CrossRef CAS PubMed; (c) N. Wiberg, T. Blank, K. Amelunxen, H. Nöth, H. Schnöckel, E. Baum, A. Purath and D. Fenske, Eur. J. Inorg. Chem., 2002, 341–350 CrossRef CAS; (d) N. Wiberg, T. Blank, H. Lemer, H. Nöth, T. Habereder and D. Fenske, Z. Naturforsch., 2001, 56b, 652–658 Search PubMed.
- T. Chu, I. Korobkov and G. I. Nikonov, J. Am. Chem. Soc., 2014, 136, 9195–9202 CrossRef CAS PubMed.
- Unpublished compound from our group which is prepared from LLi (L = PhC(NtBu)2) and AlHCl2.
- S. Khoo, Y.-L. Shan, M.-C. Yang, Y. Li, M.-D. Su and C.-W. So, Inorg. Chem., 2018, 57, 5879–5887 CrossRef CAS PubMed.
- Y. Xiong, S. Yao and M. Driess, Chem. – Eur. J., 2012, 18, 3316–3320 CrossRef CAS PubMed.
- M. Söldner, A. Schier and H. Schmidbaur, Inorg. Chem., 1997, 36, 1758–1763 CrossRef.
- F. M. Mück, J. A. Baus, R. Bertermann, C. Burschka and R. Tacke, Organometallics, 2016, 35, 2583–2588 CrossRef.
- S. Khoo, H.-Y. Yeong, Y. Li, R. Ganguly and C.-W. So, Inorg. Chem., 2015, 54, 9968–9975 CrossRef CAS PubMed.
- J. A. Cabeza, P. García-Álvare and L. González-Álvarez, Chem. Commun., 2017, 53, 10275–10278 RSC.
- S. J. Bonyhady, D. Collis, G. Frenking, N. Holzmann, C. Jones and A. Stasch, Nat. Chem., 2010, 2, 865–869 CrossRef CAS PubMed.
- S. Chen, B. Li, X. Wang, Y. Huang, J. Li, H. Zhu, L. Zhao, G. Frenking and H. W. Roesky, Chem. – Eur. J., 2017, 23, 13633–13637 CrossRef CAS PubMed.
- Z. Yang, Y. Yi, M. Zhong, S. De, T. Mondal, D. Koley, X. Ma, D. Zhang and H. W. Roesky, Chem. – Eur. J., 2016, 22, 6932–6938 CrossRef CAS PubMed.
- BrukerApex CCD, SAINTv8.30C, ed. Bruker AXS Inst. Inc., Bruker AXS Inc., WI, USA, Madison, 2013 Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., 2015, A71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., 2015, C71, 3–8 CrossRef PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, analytical data, X-ray structure determination, and computational details. CCDC 1875009 (1), 1875010 (2) and 1875008 (3) contain the supplementary crystallographic data for this paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc10124h |
‡ Crystal data for 1 at 100(2) K: C30H54Al2N4Si4, Mr = 637.09 g mol−1, 0.210 × 0.150 × 0.150 mm, monoclinic, P21/c, a = 13.559(2) Å, b = 8.253(2) Å, c = 17.689(3) Å, β = 103.31(2), V = 1926.3(7) Å3, Z = 2, μ(Mo Kα) = 0.224 mm−1, θmax = 26.4°, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 188 reflections measured, 3950 independent (Rint = 0.0532), R1 = 0.0410 [I > 2σ(I)], wR2 = 0.1143 (all data), res. density peaks: 0.425/−0.266 e A−3, CCDC: 1875009. Crystal data for 2 at 100(2) K: C36H64Al2N4Si2·0.5 (C7H8), Mr = 709.11 g mol−1, 0.260 × 0.170 × 0.100 mm, monoclinic, P21/c, a = 18.949(2) Å, b = 11.775(2) Å, c = 20.216(3) Å, β = 102.27(2), V = 4407.6(11)Å3, Z = 4, μ(Mo Kα) = 0.150 mm−1, θmax = 26.5°, 86 188 reflections measured, 3950 independent (Rint = 0.0532), R1 = 0.0410 [I > 2σ(I)], wR2 = 0.1143 (all data), res. density peaks: 0.425/−0.266 e A−3, CCDC: 1875009. Crystal data for 2 at 100(2) K: C36H64Al2N4Si2·0.5 (C7H8), Mr = 709.11 g mol−1, 0.260 × 0.170 × 0.100 mm, monoclinic, P21/c, a = 18.949(2) Å, b = 11.775(2) Å, c = 20.216(3) Å, β = 102.27(2), V = 4407.6(11)Å3, Z = 4, μ(Mo Kα) = 0.150 mm−1, θmax = 26.5°, 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568 reflections measured, 9064 independent (Rint = 0.0665), R1 = 0.0368 [I > 2σ(I)], wR2 = 0.0921 (all data), res. density peaks 0.276/−0.211 e A−3, CCDC: 1875010. Crystal data for 3 at 100(2) K: C48H74Al2N4Si2, Mr = 817.25 g mol−1, 0.195 × 0.150 × 0.144 mm, triclinic, P 568 reflections measured, 9064 independent (Rint = 0.0665), R1 = 0.0368 [I > 2σ(I)], wR2 = 0.0921 (all data), res. density peaks 0.276/−0.211 e A−3, CCDC: 1875010. Crystal data for 3 at 100(2) K: C48H74Al2N4Si2, Mr = 817.25 g mol−1, 0.195 × 0.150 × 0.144 mm, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.039(2) Å, b = 10.144(2) Å, c = 13.835(3) Å, α = 103.40(2), β = 97.39(2), γ = 114.85(3), V = 1202.2(5) Å3, Z = 1, μ(Ag Kα) = 0.083 mm−1, θmax = 20.5°, 46 , a = 10.039(2) Å, b = 10.144(2) Å, c = 13.835(3) Å, α = 103.40(2), β = 97.39(2), γ = 114.85(3), V = 1202.2(5) Å3, Z = 1, μ(Ag Kα) = 0.083 mm−1, θmax = 20.5°, 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 064 reflections measured, 4929 independent (Rint = 0.0469), R1 = 0.0370 [I > 2σ(I)], wR2 = 0.0955 (all data), res. density peaks: 0.313/−0.335 e A−3, CCDC: 1875008. The data were integrated with SAINT.33 A multi-scan absorption correction was applied using SADABS.34 The structures were solved by SHELXT35 and refined on F2 using SHELXL36 in the graphical user interface ShelXle.37 064 reflections measured, 4929 independent (Rint = 0.0469), R1 = 0.0370 [I > 2σ(I)], wR2 = 0.0955 (all data), res. density peaks: 0.313/−0.335 e A−3, CCDC: 1875008. The data were integrated with SAINT.33 A multi-scan absorption correction was applied using SADABS.34 The structures were solved by SHELXT35 and refined on F2 using SHELXL36 in the graphical user interface ShelXle.37 |
| This journal is © The Royal Society of Chemistry 2019 |