 Open Access Article
Open Access ArticleThermochemically stable ceramic composite membranes based on Bi2O3 for oxygen separation with high permeability†
Wen
Xing
 *,
Patricia A.
Carvalho
,
Jonathan M.
Polfus
*,
Patricia A.
Carvalho
,
Jonathan M.
Polfus
 and
Zuoan
Li
and
Zuoan
Li
SINTEF Industry, Sustainable Energy Technology, Pb. 124 Blindern, 0314 Oslo, Norway. E-mail: wen.xing@sintef.no
First published on 5th March 2019
Abstract
Ceramic oxygen separation membranes can be utilized to reduce CO2 emissions in fossil fuel power generation cycles based on oxy-fuel combustion. State-of-the-art oxygen permeable membranes based on Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) offer high oxygen permeability but suffer from long-term instability, especially in the presence of CO2. In this work, we present a novel ceramic composite membrane consisting of 60 vol% (Bi0.8Tm0.2)2O3−δ (BTM) and 40 vol% (La0.8Sr0.2)0.99MnO3−δ (LSM), which shows not only comparable oxygen permeability to that of BSCF but also outstanding long-term stability. At 900 °C, oxygen fluxes of 1.01 mL min−1 cm−2 and 1.33 mL min−1 cm−2 were obtained for membranes with thicknesses of 1.35 mm and 0.75 mm, respectively. Moreover, significant oxygen fluxes were obtained at temperatures down to 600 °C. A stable operation of the membrane was demonstrated with insignificant changes in the oxygen flux at 750 °C for approx. one month and at 700 °C with 50% CO2 as the sweep gas for more than two weeks.
Dense ceramic oxygen permeable membranes can separate oxygen from air at temperatures above 600 °C with higher energy efficiency than that in cryogenic distillation or pressure swing adsorption, especially when thermally and/or chemically integrated in high temperature processes.1 The separated oxygen can be used for gasification of coal and biomass, and in oxy-fuel combustion processes for power generation with carbon capture.2 Besides their applications in the energy sector, ceramic oxygen permeable membranes can also be used in small scale oxygen production or catalytic membrane reactors for the partial oxidation of methane to syngas, and for the production of chemicals.3,4 It is particularly beneficial with chemical integration of the membranes which then must be CO2-tolerant for most applications, e.g., oxy-fuel with recirculated flue gas on the permeate side.5
Dense ceramic membranes rely on the ambipolar transport of both oxide ions and electrons or holes, i.e., mixed ionic and electronic conduction (MIEC). The highest oxygen permeation rates for this type of mixed conducting membranes were found in Ba1−xSrxFeO3−δ and SrCo1−xFexO3−δ based perovskites such as Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF). The oxygen flux of asymmetric and thin BSCF membranes can reach ∼10 mL min−1 cm−2.6,7 However, BSCF suffers from several structural and chemical instability issues that hinder the material's practical application as membranes. BSCF undergoes a phase transition from cubic to hexagonal that leads to its decomposition into two phases when annealed below 850 °C.8–10 Due to the basicity of barium, BSCF exhibits severe reactivity towards CO2 which significantly reduces the membrane performance.11 The high thermal expansion coefficient of BSCF (approx. 20 × 10−6 K−1) also limits its integration with other components in modules or reactors.12 Membrane materials that are stable towards CO2 such as SrFe0.9Ta0.1O3−δ13 and (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δCl0.114 exhibit lower oxygen fluxes of 0.3 and 0.8 mL min−1 cm−2 at 900 °C for disc membranes of 1 mm and 0.6 mm thicknesses, respectively, as compared to BSCF.
Ambipolar transport of oxide ions and electrons can alternatively be achieved by means of a ceramic composite comprising an oxide ion conducting electrolyte and an electronic conducting oxide. Chemical compatibility between the phases is crucial, and the interphase between the two materials plays an important role in determining the membrane performance and stability. For instance, segregation or reaction along the heterophase boundaries may lead to the formation of blocking phases that limit the overall oxide ion and/or electronic transport, while sufficient adherence between the phases is required.
The ambipolar conductivity and oxygen flux of the composite membranes is usually limited by the oxide ion conducting phase since the conductivity of a percolating electronic conducting phase can be significantly higher. Fluorite structured oxides have been selected due to their excellent chemical stability and relatively high oxide ion conductivity. Composites of acceptor doped CeO2 with electronic or mixed conducting oxides have been extensively studied, and present moderate oxygen fluxes as compared to BSCF with a similar thickness.15–26 Composite membranes based on stabilised ZrO2 show even lower oxygen fluxes.27–29
In comparison to CeO2 and ZrO2 based oxide ion conductors, δ-Bi2O3 shows a higher oxide ion conductivity30 and the high temperature phase can be stabilized at lower temperatures by doping with lanthanide elements.31–34 Previous studies have focused on composite membranes based on doped Bi2O3 mixed with noble metals and relatively high oxygen fluxes were obtained.35–37 However, these cermet membranes exhibited high and unsuitable thermal expansion, and the use of noble metals may be an issue for their applicability due to the material cost.
Here we present a composite oxygen separation membrane based on Tm-doped Bi2O3 and p-type conducting Sr-doped LaMnO3. Among the lanthanide dopants for Bi2O3, thulium was chosen due to its suitable ionic radius for attaining the cubic δ-Bi2O3 phase which exhibits high phase stability at lower temperatures and high oxide ion conductivity.34
The composite pellet membranes were synthesized by the solid-state reaction method. Bi2O3 (99.9%, Sigma Aldrich) and 20 molar percent of Tm2O3 (99.9%, Sigma Aldrich) were weighed and mixed in an agate mortar. The mixture was calcinated at 750 °C for 10 hours to form a single phase (Bi0.8Tm0.2)2O3−δ. (La0.8Sr0.2)0.99MnO3−δ (LSM) was synthesized by spray pyrolysis (Cerpotech, Norway). The BTM and LSM powders were mixed in an agate mortar with a volume ratio of 60% to 40%, respectively, to ensure percolation of both phases. Green pellets (∅ = 21 mm) were pressed and sintered at 1000 °C for 10 hours resulting in membranes with a relative density of 93%. The samples were characterised by scanning electron microscopy (SEM) using an FEI Nova NaonSEM 650 apparatus. Phase boundary observations were carried out by scanning transmission electron microscopy (STEM) using a DCOR Cs probe-corrected FEI Titan G2 60-300 instrument with 0.08 nm of nominal spatial resolution (details can be found in ESI†). The thermal expansion coefficient of the composite membrane was measured using a NETZSCH Dilatometer 402E.
The disc samples were polished using an SiC paper down to 2 μm roughness. Gold ring sealing gaskets of 13 mm in diameter were polished using a diamond paste down to 250 nm roughness. The samples were mounted in a ProboStat measurement cell (NORECS, Norway) and sealed to an alumina riser tube upon heating the setup to 950 °C. The He leakage to the sweep was monitored by gas chromatography (GC, Varian CP-4900) using helium and oxygen mixtures as feed gases. Throughout the measurements after the sealing was achieved, no helium was detected in the sweep gas stream within the detection limit of the GC (1 ppm). Therefore, the oxygen flux (jO2) was obtained from the concentration of oxygen measured by GC in the sweep (cO2) according to the following equation:
| jO2 = cO2Fsweep/A | (1) |
The SEM cross-section images shown in Fig. 1 show dark LSM grains immersed in large grained BTM, forming interconnected networks. The grain growth of LSM can be observed after the permeation test for about 2 months, and no secondary phases were observed by XRD (Fig. S5, ESI†).
 | ||
| Fig. 1 Fracture cross-section of the composite membrane before (left) and after (right) a long term flux test by SEM. The darker grains are LSM and the brighter phase is BTM. | ||
The detailed structural and chemical properties of the interface between BTM and LSM were investigated by STEM. The observations revealed high interfacial adhesion (Fig. 2(a)), and EDS line profiles across the interfaces showed a substantial cation interdiffusion within about 40 nm (Fig. 2(b)). Fig. 2(c) shows that the interfaces were atomically smooth and coherent, which can be ascribed to the similar interatomic distances in the two structures (see XRD patterns in ESI†). The atomic resolution EDS mapping confirms the La substitution by Sr in the LSM phase (Fig. 2(d)), while no chemical segregation at the interfaces from either BTM or LSM could be detected (see Fig. 2(e)). The pristine interfaces in the composite observed by TEM may enhance an interfacial oxide ion diffusion in LSM as reported recently.38
Fig. 3 shows the measured oxygen fluxes of the BTM–LSM membranes with different thicknesses as a function of inverse temperature in comparison to the literature data for BSCF and composites based on the acceptor-doped CeO2 and ZrO2 with similar thicknesses. At high temperatures, the thinner 0.75 mm membrane shows a higher oxygen flux than the thicker 1.35 mm membrane. The oxygen fluxes were similar at temperatures below 750 °C, which indicate limiting surface kinetics at lower temperatures.‡ Overall, the BTM–LSM composite membranes show a comparable oxygen flux to that of the BSCF membranes and a higher oxygen flux than the CeO2 and ZrO2 based composite membranes that can be ascribed to the higher oxide ion conductivity in the acceptor-doped Bi2O3.
 | ||
| Fig. 3 Oxygen flux as a function of inverse temperature for the BTM–LSM compared to BSCF and acceptor-doped CeO2 and ZrO2 composite membranes. The oxygen content in the feed gas was normalised to 50%. Flux data was included for BSCF membranes with thicknesses of 1.8 mm10 and 1 mm,39 composites of Ce0.85Gd0.1Cu0.05O2−δ (CGCO) and La0.6Ca0.4FeO3−δ (LCF),21 Sm0.2Ce0.8O1.9−δ (SDC) and Y0.8Ca0.2Cr0.8Co0.2O3−δ (YCCC),23 and (ZrO2)0.89(Y2O3)0.01(Sc2O3)0.10 (ScYSZ) and Al0.02Zn0.98O1.01 (AZO).29 | ||
Based on Wagner transport theory for mixed conducting membranes and assuming no surface kinetics limitations, the oxygen flux is proportional to the chemical potential gradient of oxygen across the membrane:
 | (2) |
The long-term flux stability of the BTM–LSM membranes was investigated for up to 1 month at 750 °C and with 50% CO2 in the sweep for more than 400 hours at 700 °C. First, the O2 flux was essentially unchanged with 50% CO2 in the sweep throughout 900 °C to 600 °C (Fig. S4, ESI†). Subsequently, the membrane was kept with 50% CO2 in the sweep at 700 °C, and Fig. 5 shows that the flux exhibited a moderate decrease of about 10% during the initial 100 hours, while it remained stable for the remaining 300 hours. Finally, the flux was measured at 750 °C with argon sweep for about 700 hours where the membrane showed very good stability with insignificant changes in the oxygen flux. Carbonate formation was not observed by SEM or XRD (Fig. S5, ESI†).
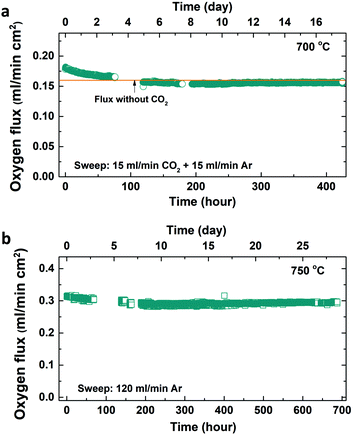 | ||
| Fig. 5 (a) Oxygen flux with 50% CO2 sweep at 700 °C for more than 400 hours and (b) subsequently at 750 °C with Ar sweep for approximately one month. | ||
The thermal expansion coefficient of BTM–LSM at temperatures between 300 °C and 900 °C in air was 12.5 × 10−6 °C−1 upon heating and 11.5 × 10−6 °C−1 during cooling. BTM–LSM composite membranes thereby show significantly lower thermal expansion than BSCF which is crucial for system integration of the membranes in reactors or modules.
Further improvements in the oxygen permeation performance of the BTM–LSM system may be achieved by optimization of the Bi2O3 dopant and microstructure of the composite, while supported asymmetric membrane architectures with catalytic layers should be pursued for the future membrane development.
Ceramic composite membranes based on oxide ion conducting Tm-doped Bi2O3 and the p-type conducting Sr-doped LaMnO3 exhibit good chemical compatibility and comparable oxygen permeation rates compared to BSCF. Moreover, the BTM–LSM membranes show excellent stability at working temperatures in the presence of CO2 containing atmospheres. The thermal expansion coefficient of the composite is significantly lower than that of BSCF, and suitable for integration with other components. Overall, composite membranes based on BTM–LSM are outstanding in many aspects compared to the current state-of-the-art material systems, and may support the future use of the ceramic oxygen separation membrane in clean energy applications.
The authors acknowledge financial support from the Research Council of Norway (RCN) under the CLIMIT program (MOC-OTM 268450). Ove Paulsen is acknowledged for TEC measurements.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Zhang, J. Sunarso and S. Liu, Chem. Soc. Rev., 2017, 46, 2941–3005 RSC.
- R. Kneer, D. Toporov, M. Forster, D. Christ, C. Broeckmann, E. Pfaff, M. Zwick, S. Engels and M. Modigell, Energy Environ. Sci., 2010, 3, 198–207 RSC.
- A. Thursfield and I. S. Metcalfe, J. Mater. Chem., 2004, 14, 2475–2485 RSC.
- S. S. Hashim, A. R. Mohamed and S. Bhatia, Renewable Sustainable Energy Rev., 2011, 15, 1284–1293 CrossRef CAS.
- M. A. Habib, H. M. Badr, S. F. Ahmed, R. Ben-Mansour, K. Mezghani, S. Imashuku, G. J. la O', Y. Shao-Horn, N. D. Mancini, A. Mitsos, P. Kirchen and A. F. Ghoneim, Int. J. Energy Res., 2011, 35, 741–764 CrossRef CAS.
- X. Zhu, S. Sun, Y. Cong and W. Yang, J. Membr. Sci., 2009, 345, 47–52 CrossRef CAS.
- S. Baumann, J. M. Serra, M. P. Lobera, S. Escolástico, F. Schulze-Küppers and W. A. Meulenberg, J. Membr. Sci., 2011, 377, 198–205 CrossRef CAS.
- S. Švarcová, K. Wiik, J. Tolchard, H. J. M. Bouwmeester and T. Grande, Solid State Ionics, 2008, 178, 1787–1791 CrossRef.
- M. Arnold, T. M. Gesing, J. Martynczuk and A. Feldhoff, Chem. Mater., 2008, 20, 5851–5858 CrossRef CAS.
- Z. Shao, W. Yang, Y. Cong, H. Dong, J. Tong and G. Xiong, J. Membr. Sci., 2000, 172, 177–188 CrossRef CAS.
- M. Arnold, H. Wang and A. Feldhoff, J. Membr. Sci., 2007, 293, 44–52 CrossRef CAS.
- S. McIntosh, J. F. Vente, W. G. Haije, D. H. A. Blank and H. J. M. Bouwmeester, Chem. Mater., 2006, 18, 2187–2193 CrossRef CAS.
- J. Zhu, S. Guo, Z. Chu and W. Jin, J. Mater. Chem. A, 2015, 3, 22564–22573 RSC.
- J. Xue, J. Li, L. Zhuang, L. Chen, A. Feldhoff and H. Wang, Chem. Eng. J., 2018, 347, 84–90 CrossRef CAS.
- V. V. Kharton, A. V. Kovalevsky, A. P. Viskup, A. L. Shaula, F. M. Figueiredo, E. N. Naumovich and F. M. B. Marques, Solid State Ionics, 2003, 160, 247–258 CrossRef CAS.
- A. J. Samson, M. Søgaard and P. Vang Hendriksen, J. Membr. Sci., 2014, 470, 178–188 CrossRef CAS.
- X. F. Zhu and W. S. Yang, AIChE J., 2008, 54, 665–672 CrossRef CAS.
- T. Chen, H. Zhao, N. Xu, Y. Li, X. Lu, W. Ding and F. Li, J. Membr. Sci., 2011, 370, 158–165 CrossRef CAS.
- Y. Lin, S. Fang, D. Su, K. S. Brinkman and F. Chen, Nat. Commun., 2015, 6, 6824 CrossRef CAS PubMed.
- S. Cheng, M. Sogaard, L. Han, W. Zhang, M. Chen, A. Kaiser and P. V. Hendriksen, Chem. Commun., 2015, 51, 7140–7143 RSC.
- W. Fang, F. Liang, Z. Cao, F. Steinbach and A. Feldhoff, Angew. Chem., Int. Ed., 2015, 54, 4847–4850 CrossRef CAS PubMed.
- S. Fang, C. Chen and L. Winnubst, Solid State Ionics, 2011, 190, 46–52 CrossRef CAS.
- K. J. Yoon and O. A. Marina, J. Membr. Sci., 2016, 499, 301–306 CrossRef CAS.
- K. Partovi, C. H. Rüscher, F. Steinbach and J. Caro, J. Membr. Sci., 2016, 503, 158–165 CrossRef CAS.
- H. Li, Y. Liu, X. Zhu, Y. Cong, S. Xu, W. Xu and W. Yang, Sep. Purif. Technol., 2013, 114, 31–37 CrossRef CAS.
- H. Luo, H. Jiang, T. Klande, F. Liang, Z. Cao, H. Wang and J. Caro, J. Membr. Sci., 2012, 423–424, 450–458 CrossRef CAS.
- S. Lia, W. Jin, N. Xu and J. Shi, J. Membr. Sci., 2001, 186, 195–204 CrossRef CAS.
- W. He, H. Huang, J.-F. Gao, L. Winnubst and C.-S. Chen, J. Membr. Sci., 2014, 452, 294–299 CrossRef CAS.
- S. Pirou, J. M. Bermudez, P. V. Hendriksen, A. Kaiser, T. R. Reina, M. Millan and R. Kiebach, J. Membr. Sci., 2017, 543, 18–27 CrossRef CAS.
- P. Shuk, H. D. Wiemhöfer, U. Guth, W. Göpel and M. Greenblatt, Solid State Ionics, 1996, 89, 179–196 CrossRef CAS.
- H. T. Cahen, T. G. M. Van Den Belt, J. H. W. De Wit and G. H. J. Broers, Solid State Ionics, 1980, 1, 411–423 CrossRef CAS.
- S. Boyapati, E. D. Wachsman and N. Jiang, Solid State Ionics, 2001, 140, 149–160 CrossRef CAS.
- M. Drache, P. Roussel and J.-P. Wignacourt, Chem. Rev., 2007, 107, 80–96 CrossRef CAS PubMed.
- A. Dapčević, D. Poleti, J. Rogan, A. Radojković, M. Radović and G. Branković, Solid State Ionics, 2015, 280, 18–23 CrossRef.
- J. Kim and Y. S. Lin, J. Membr. Sci., 2000, 167, 123–133 CrossRef CAS.
- K. Kobayashi and T. Tsunoda, Solid State Ionics, 2004, 175, 405–408 CrossRef CAS.
- K. Wu, S. Xie, G. S. Jiang, W. Liu and C. S. Chen, J. Membr. Sci., 2001, 188, 189–193 CrossRef CAS.
- J. M. Polfus, B. Yildiz and H. L. Tuller, Phys. Chem. Chem. Phys., 2018, 20, 19142–19150 RSC.
- S. Baumann, F. Schulze-Küppers, S. Roitsch, M. Betz, M. Zwick, E. M. Pfaff, W. A. Meulenberg, J. Mayer and D. Stöver, J. Membr. Sci., 2010, 359, 102–109 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc10077b |
| ‡ Additional evidence of limiting surface kinetics can be found in Fig. S2 in the ESI.† |
| § The membrane performance does not follow exactly eqn (2) as surface kinetics plays an important role in this case. A plot of the oxygen fluxes as a function of oxygen partial pressure gradient can be found in Fig. S3 in the ESI.† |
| This journal is © The Royal Society of Chemistry 2019 |


