Catalyst-free hydrothiolation of alkynes with dithiocarbamic acids†
Abstract
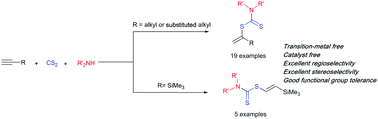
A highly regio- and stereocontrolled procedure for the synthesis of vinyl dithiocarbamates via catalyst-free hydrothiolation of unactivated alkynes with in situ prepared dithiocarbamic acids with total atom economy is reported. While unactivated terminal alkynes afforded the Markovnikov adducts with excellent regioselectivity, ethynyltrimethylsilane furnished the E-selective anti-Markovnikov adducts with excellent regio- and stereoselectivity. In addition, the obtained vinyl dithiocarbamates were applied as efficient precursors for the synthesis of secondary thiols and α-hydroxythioamides.


 Please wait while we load your content...
Please wait while we load your content...