Photoactive chelates for radiolabelling proteins†
Abstract
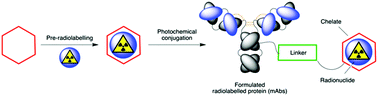
Photochemical reactions are an attractive foundation for the synthesis of radiolabelled antibodies, immunoglobulin fragments and other proteins/peptides. The synthesis, 68Ga-radiochemistry and photochemical reactivity of three macrocyclic chelates functionalised with an arylazide group is described. Experiments with trastuzumab confirmed that photoradiochemistry facilitates fast, one-pot synthesis of radiolabelled proteins.


 Please wait while we load your content...
Please wait while we load your content...