Intense upconversion red emission from Gd-doped NaErF4:Tm-based core/shell/shell nanocrystals under 980 and 800 nm near infrared light excitations†
Abstract
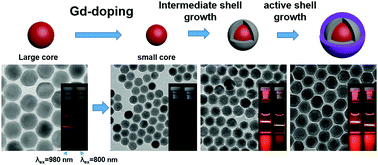
Bright and single band red-emitting NaErF4:Tm,Gd-based upconversion nanophosphors (UCNPs) were successfully synthesized by the formation of a core/intermediate shell/active shell structure. The NaErF4:Tm,Gd/NaYF4:Ca,Yb/NaYF4:Nd,Yb core/shell/shell UCNPs exhibit intense red light under both 980 and 800 nm near infrared light excitations.


 Please wait while we load your content...
Please wait while we load your content...