Tirapazamine-embedded polyplatinum(IV) complex: a prodrug combo for hypoxia-activated synergistic chemotherapy†
Dongbo
Guo
ab,
Shuting
Xu
a,
Wumaier
Yasen
a,
Chuan
Zhang
 *a,
Jian
Shen
*c,
Yu
Huang
a,
Dong
Chen
*a,
Jian
Shen
*c,
Yu
Huang
a,
Dong
Chen
 a and
Xinyuan
Zhu
a and
Xinyuan
Zhu
 *a
*a
aSchool of Chemistry and Chemical Engineering, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuant Road, Shanghai 200240, China. E-mail: chuanzhang@sjtu.edu.cn; xyzhu@sjtu.edu.cn
bSouth China Institute of Collaborative Innovation, School of Materials Science and Engineering, South China University of Technology, Dongguan, 523808, China
cJiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of Biomedical Materials, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210046, China. E-mail: shenjianbio@hotmail.com
First published on 15th November 2019
Abstract
Despite the great advances achieved in hypoxia-associated tumor therapy, the efficacy of hypoxia-activated prodrugs alone is usually limited owing to the moderate oxygen supply at the tumor area. Herein, we develop a polymerized platinum(IV) compound-based nanogel (polyprodrug) containing a bioreductive and hypoxia-activated prodrug (tirapazamine, TPZ) as a prodrug combo (polyprodrug@TPZ) for synergistic chemotherapy. Upon exposure to the tumor microenvironment, platinum(IV) moieties in the polyprodrug are reduced to platinum(II) species, which significantly upregulates the expression of NADPH oxidases (NOXs) to accelerate oxygen (O2) depletion and promote reactive oxygen species (ROS) production, as confirmed by reverse transcription-PCR (RT-PCR) and fluorescence probes. In the exaggerated hypoxia environment, highly cytotoxic radicals are generated due to TPZ activation, which serve as second antitumor agents working together with platinum(II) species in synergistic chemotherapy. With the rational design of nanosized architecture, the platinum(IV)-based polyprodrug@TPZ complex exhibits the advantages of redox-responsive drug release, superior tumor accumulation, and long-term circulation during the synergistic antitumor treatment in a mouse model. These results indicate that combination of an oxygen depletion prodrug and hypoxia-activated antitumor agents would serve as a promising strategy to realize a better synergistic chemotherapy.
Introduction
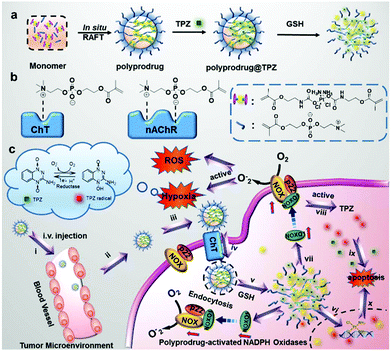
Hypoxia, severe oxygen deprivation, has been considered as a hallmark feature of solid tumors owing to irregular blood vasculature and disordered cell proliferation.1–3 Hypoxic domains normally play essential roles in resistance during conventional cancer therapies, such as radiotherapy, chemotherapy, and photo-dynamic therapy.3 Although hypoxia-activated prodrugs have been regarded as a smart method for hypoxic tumor therapy,4–13 antitumor efficacy of hypoxia-activated prodrugs alone is usually impeded due to the moderate O2 supply at the tumor site. Thus, inhibiting O2-supply or enhancing O2-depletion at the tumor area provides a solution to exaggerate the hypoxic microenvironment and enhance the cytotoxicity of hypoxia-activated prodrugs.In the clinic, platinum-based drugs are one of the most important chemotherapeutic drugs for single or combination cancer therapies against a wide range of cancers.14,15 Specifically, platinum drugs play a vital role in ROS generation for enhanced antitumor effects.16,17 In the meantime, platinum drugs can induce a family of oxidases denominated as NOXs in cancer cells,18,19 wherein NOXs have the ability to transport electrons across the plasma membrane. Correspondingly, an O2˙− could be generated by an oxygen molecule accepting a donated electron. This process is beneficial to enhancing cytotoxicity of the hypoxia-activated prodrug by reducing O2 concentration.20,21 Based on the platinum drug induced NOX expression and the NOX-enhanced consumption of endogenous oxygen, we speculate that the combination of an oxygen depletion chemodrug and hypoxia-activated prodrugs may serve as a promising strategy for a synergistic antitumor therapy.
In this study, a novel polyplatinum(IV) complex with capabilities of promoting NOX-associated hypoxia and releasing two antitumor agents (bioreductive platinum(II) species and hypoxia-activated prodrug tirapazamine) was rationally designed for synergistic chemotherapy (Fig. 1). In detail, a Pt(IV)-based prodrug monomer (PPM) was prepared according to our previous method.22 Because of its symmetrical axial bis-urethan ethyl methacrylate ligands, PPM is easily polymerized into diverse functional polymers.23–27 As nicotinic acetylcholine receptor (nAChRs) and choline transporter (ChTs) are expressed in non-small cell lung cancer (NSCLC),28–30 their acetylcholine and choline analogues should improve the delivery ability from the blood circulation to the tumor. Thus, a choline or acetylcholine analogue, 2-methacryloyloxyethyl phosphoryl-choline (MPC), was employed to copolymerize with PPM to prepare polyplatinum(IV) complex-based nanogels (abbreviated as polyprodrug), which may efficiently transport the drug to tumor sites by interacting with ChTs in a similar way to choline.31,32 After encapsulating the hypoxia-activated prodrug TPZ, the as-prepared complex (abbreviated as polyprodrug@TPZ) overcomes the inevitable drawbacks of small molecule drugs such as severe side effects, insufficient water-solubility, low cellular uptake efficiency, and nonspecific delivery.33,34 After exposure to the tumor microenvironment, the platinum(II) species, the product of reduction from the platinum(IV) moiety in the nanogel complex, significantly upregulates the expression of NOXs to accelerate oxygen depletion and reactive oxygen species (ROS) production. Importantly, the hypoxic microenvironment in the tumor, partially caused by NOX-induced oxygen depletion, activates the cytotoxicity of TPZ, which serves as a second antitumor agent working together with platinum(II) species in the synergistic chemotherapy.
Experimental
Materials
Cisplatin, 1,3-diphenylisobenzofuran (DPBF), 2-isocyantoethyl methacrylate, 4-dimethylaminopyridine (DMAP), anhydrous dimethyl sulfoxide (DMSO), 2-methacryloyloxy ethyl phosphorylcholine (MPC), 2,6-di-tert-butyl-4-methylphenol (BHT), 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT), diphenyleniodonium chloride (DPI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT), azobisisobutyronitrile (AIBN), methanol, anhydrous dimethylformamide (DMF), sodium hydrosulfite, tirapazamine (TPZ), lysozyme, pepsin, trypsin and other chemicals were purchased from Sigma-Aldrich. 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) and terminal deoxy-nucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays were purchased from Beyotime (one step TUNEL apoptosis assay kit, China). Trizol reagent and anti-HIF-1α antibody were purchased from Invitrogen Life Technologies. All the secondary antibodies were purchased from Abcam (Abcam, Cambridge, MA, USA). All materials were used without further purification unless indicated otherwise.RT-PCR and real-time PCR
A549 cells were incubated with polyprodrug (20 μM Pt) for set time periods. TRIzol was used to isolate total RNA. Then, single-stranded cDNA was synthesized by reverse transcription from total RNA. Then, PCR using TaqDNA polymerase (Platinum™ Taq DNA polymerase high fidelity, Invitrogen, USA) was performed for 40 cycles following this process: 95 °C for 25 s, 65 °C for 25 s, and 70 °C for 35 s. Subsequently, 1.2% agarose gel was used to separate the PCR products (15 μL), which were visualized under UV light. PCR was used to amplify the sequences of primers as follows: NOX1 (forward, 5′-CTT GGG TCA ACA TTG GCC TGT-3′; reverse, 5′-GCT TGT GGA AGG TGA GGT TGT-3′, NM_007052.4); NOX3 (forward, 5′-TTG TGG CAC ACT TGT TCA ACC-3′; reverse, 5′-TCA CAC GCA TAC AAG ACC ACA GGA-3′, NM_000397.3); NOX2 (forward, 5′-CTG AAC GAA TTG TAC GTG GGC-3′; reverse, 5′-ACC CAC TAT CCA TTT CCA AGT CAT-3′, NM_000397.3); NOX4 (forward, 5′-AGG AGG GCT GCT GAA GTA TCA-3′; reverse, 5′-ACC CAC TAT CCA TTT CCA AGT CAT-3′, NM_001143836.2); NOXO1 (forward, 5′-GTG GCT GGT GGA GAA CGA AGA-3′; reverse, 5′-CGC GGT CTG ACG TTT CCAA-3′, NM_001267721.1); NOXA1 (forward, 5′-CAC TTG GAG CCC GTG GAT TT-3′; reverse, 5′-GTT TGC CAA CTT GCT CCA CCT-3′, NM_001256067.1); p22phox (forward, 5′-ATT GTG GCG GGC GTG TTT-3′; reverse, 5′-CAC AGC CGC CAG TAG GTA GAT G-3′, NM_000101.3); p47phox (forward, 5′-CCC CAC GGA CAA CCA GAC AAA-3′; reverse, 5′-TGA CAG AAC CAC CAA CCG CTC-3′, NM_000265.5); p67phox (forward, 5′-AGC TGT TTG CCT GTG AGG TGT-3′; reverse, 5′-GCT TCC AGA CAC ACT CCA TCG-3′, NM_000433.3); and β-actin (forward, 5′-CAC CCA GCA CAA TGA AGA TCA AGA T-3′; reverse, 5′-CCA GTT TTT AAA TCC TGA GTC AAG C-3′, NM_001101). Real-time PCR analysis using a LightCycler rapid thermal cycler system (Roche Diagnostics Ltd, Lewes, UK) was performed for quantitative analysis of mRNAs. Reactions were carried out in 25 μL of primers (0.5 μM) with MgCl2 concentration between 3 and 6 mM. Taq DNA polymerase, buffer, and nucleotides were mixed in the LightCycler 480 SYBR Green I mix. β-Actin was used to standardize the copy numbers of mRNA.MTT assays
Cytotoxicity profiles of TPZ, polyprodrug@TPZ in normoxia, polyprodrug@TPZ in hypoxia against non-small cell lung cancer (A549 cells), and polyprodrug@TPZ against NOX1 knockdown A549 cells were measured by MTT assay. A 96 well plate (8000 cells per well) was seeded with A549 cells in 200 μL of RPMI treated with variable concentrations of TPZ or polyprodrug@TPZ for 24 h at 37 °C. For the NOX1 siRNA transfected group, A549 cells were pretreated with 100 nM NOX1 siRNA at 34 °C for 36 h, followed with polyprodrug@TPZ for 24 h at 37 °C. The A549 cells were then treated with 200 μL of fresh medium containing MTT (0.8 mg mL−1) for 3 h at 37 °C. An anaero Pack-Anaero (Mitsubishi Gas Chemical Co. Inc., Japan) was used to provide in vitro environments to mimic the tumor hypoxia. After removing the medium, each well was supplemented with 200 μL of DMSO. Then, a BioTek Synergy HT multi-detection microplate plate reader was selected to record the absorption of the purple formazan at 570 nm. Each experiment was performed in triplicate for the A549 cell line.Combination index (CI) analysis
The combination index was calculated with CalcuSyn® ver.2.0 (Biosoft, Cambridge, UK) according to the mathematical model of Chou–Talalay analyses, based on the linear dose-effect curves. Subsequently, we used the CI-isobologram equation to determine synergism and antagonism. In detail, the additive effect, synergistic effect, or antagonistic effect between the Pt complex-based drug and TPZ is indicated by CI values of 1, CI < 1, or CI > 1, respectively.ROS production
A cell membrane permeable organic probe, 2′,7′-dichlorofluorescein diacetate (DCFH-DA), was used to determine the intracellular ROS level. The highly fluorescent 2′,7′-dichlorofluorescein is activated in the presence of an oxidant, which is derived from DCFH-DA. Briefly, A549 cells were transfected with NOX1 (5 nM) siRNA or pretreated with DPI. The cells were then treated with polyprodrug for 12 h followed by incubation with 5 mM DCFH-DA dye for 15 min. The green fluorescence (excitation/emission: 488/530 nm) was measured by using confocal microscopy (LSM 710, Zeiss, Germany). DPI was used as an inhibitor of NADPH oxidases. Furthermore, A549 cells were pre-incubated with DPI or pre-transfected with NOX1 siRNA for 30 min. Then, A549 cells were treated with 20 μM polyprodrug nanogels for 12 h.Oxygen depletion rate
Oxygen depletion rate was analyzed to evaluate hypoxia induced by polyprodrug nanogels. In brief, A549 cells or NOX1 siRNA transfected A549 cells were seeded on a 6 well plate (3 × 106 cells per dish) in 3 mL of RPMI overnight, then cells were incubated with the polyprodrug nanogels (20 μM Pt) or polyprodrug + DPI (20 μM Pt and 10 μM DPI). After 12 h of incubation, paraffin was used to isolate the replaced culture medium and air. Then, oxygen depletion rate was measured with a Clark oxygen electrode (DOG-3082 oxygen dissolving meter, Shanghai Boqu Instrument Co. Limited) and expressed as a percentage of the baseline. The temperature was maintained at 37 °C during the measurement.Blood circulation time of nanogels
The experimental protocols involving animals were approved by the Animal Ethics Committee of Shanghai Jiao Tong University and performed in compliance with the Guidelines for Care and Use of Laboratory Animals of Shanghai Jiao Tong University. To verify the blood circulation time of TPZ and polyprodrug@TPZ, 200 μL of solutions was intravenously injected into Sprague-Dawley rats (SD, ∼200 g) by the tail vein with equivalent TPZ concentration at 4 mg kg−1. After being randomly assigned to two groups (n = 4), the blood samples (500 μL) were collected from the eye socket at 0.5 h, 1.0 h, 2.0 h, 8.0 h, 12.0 h, and 24.0 h post-administration, then stored at −20 °C in heparinized tubes. The plasma was obtained from the blood samples by centrifugation. High performance liquid chromatography (Agilent 1260 HPLC, USA) was used to determine the UV-vis absorption (λ = 500 nm) of drugs in the blood.Immunohistochemistry
A549 xenograft tumor-bearing male nude mice were administrated by intravenous injection with a polyprodrug@TPZ (200 μL, 2 mg mL−1) solution. The mice were randomly divided into 3 groups: 12 h post-injection group, 24 h post-injection group, and the control group. Immunohistochemistry staining was performed by collecting the tumor tissues of nude mice in these three groups and fixing for biochemical analysis. The hypoxia parts and blood vasculatures were stained with anti-pimonidazole antibody (red) and anti-CD31 antibody (green), respectively. DAPI was used to stain the nuclei of cells (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, Invitrogen) simultaneously. Subsequently, confocal scanning laser microscopy was used to visualize the fluorescence image.
5000, Invitrogen) simultaneously. Subsequently, confocal scanning laser microscopy was used to visualize the fluorescence image.
Statistics
Data are presented as mean ± S.D. Statistical analysis was carried out by using Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001, respectively).Results and discussion
Syntheses and characteristics
The Pt(IV)-based prodrug monomer (PPM) and polyprodrug nanogel were prepared according to Fig. S1.† In brief, PPM was prepared by co-mixing methacrylic acid-2-isocyanatoethyl ester with cis, cis, trans-[PtCl2(OH)2(NH3)2] via a one-step protocol (see ESI† for details).22,35 The structure of PPM was verified by using 1H NMR spectroscopy. The integration areas of protons attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH (5.98–6.14 ppm) and ammine ligands (6.30–6.77 ppm) had a ratio of around 1
CH (5.98–6.14 ppm) and ammine ligands (6.30–6.77 ppm) had a ratio of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, suggesting that the platinum(IV) compound was well conjugated with bis-urethan ethyl methacrylate groups (Fig. S2†). Then, high-performance liquid chromatography tandem mass spectrometry (HPLC-MS) was utilized to verify the successful preparation of PPM. As shown in Fig. S3,† a retention peak at about 3.76 min with a molecular weight of 645.0810 and 1289.1736 was observed, corresponding to [M + H]+ and [2M + H]+, respectively. After polymerization by reversible addition–fragmentation chain transfer (RAFT) polymerization, the chemical structure of the Pt(IV)-complex polyprodrug was also proved by using 1H NMR spectroscopy (Fig. S4†). The integration area of protons attributed to –N(CH3)3 groups (∼2.48 ppm) and –CH2–N– (∼3.06 ppm) indicated that the polyprodrug nanogels were successfully prepared by copolymerizing PPM and MPC. After synthesis of the platinum(IV)-based polyprodrug, the Pt content of the polyprodrug nanogels was confirmed by using inductively coupled plasma atomic emission spectrometry (ICP-AES), which showed a Pt content of 8.06 wt% (Table S1†). TPZ was loaded into the polyprodrug nanogels through electrostatic interactions, forming a complex (abbreviated as polyprodrug@TPZ) with a loading content up to 9.12%. The sizes and morphologies of the polyprodrug nanogels and polyprodrug@TPZ were measured by dynamic light scattering (DLS) and transmittance electron microscopy (TEM). The average diameters of polyprodrug nanogels and polyprodrug@TPZ were around 105 nm and 138 nm, respectively (Fig. S5†). Meanwhile, spherical nanoparticles were observed in the TEM image of the polyprodrug nanogels and polyprodrug@TPZ, being about 98 nm and 123 nm in size, respectively (Fig. S6† and Fig. 2a).
3, suggesting that the platinum(IV) compound was well conjugated with bis-urethan ethyl methacrylate groups (Fig. S2†). Then, high-performance liquid chromatography tandem mass spectrometry (HPLC-MS) was utilized to verify the successful preparation of PPM. As shown in Fig. S3,† a retention peak at about 3.76 min with a molecular weight of 645.0810 and 1289.1736 was observed, corresponding to [M + H]+ and [2M + H]+, respectively. After polymerization by reversible addition–fragmentation chain transfer (RAFT) polymerization, the chemical structure of the Pt(IV)-complex polyprodrug was also proved by using 1H NMR spectroscopy (Fig. S4†). The integration area of protons attributed to –N(CH3)3 groups (∼2.48 ppm) and –CH2–N– (∼3.06 ppm) indicated that the polyprodrug nanogels were successfully prepared by copolymerizing PPM and MPC. After synthesis of the platinum(IV)-based polyprodrug, the Pt content of the polyprodrug nanogels was confirmed by using inductively coupled plasma atomic emission spectrometry (ICP-AES), which showed a Pt content of 8.06 wt% (Table S1†). TPZ was loaded into the polyprodrug nanogels through electrostatic interactions, forming a complex (abbreviated as polyprodrug@TPZ) with a loading content up to 9.12%. The sizes and morphologies of the polyprodrug nanogels and polyprodrug@TPZ were measured by dynamic light scattering (DLS) and transmittance electron microscopy (TEM). The average diameters of polyprodrug nanogels and polyprodrug@TPZ were around 105 nm and 138 nm, respectively (Fig. S5†). Meanwhile, spherical nanoparticles were observed in the TEM image of the polyprodrug nanogels and polyprodrug@TPZ, being about 98 nm and 123 nm in size, respectively (Fig. S6† and Fig. 2a).
According to our previous report, the release behaviors of TPZ and Pt(II) species from polyprodrug@TPZ nanogels were evaluated in different buffer solutions.19 As shown in Fig. 2b and c, polyprodrug@TPZ was stable in PBS. Only about 11.3% and 17.3% Pt(II) species, and 21.5% and 22.1% TPZ were released in either neutral (pH = 7.4) or acidic (pH = 5.0) PBS post 50 h incubation, respectively. In contrast, when polyprodrug@TPZ nanogels were placed in a reductive medium, a sharp release curve of TPZ and Pt(II) species could be achieved, indicating that the drug release behaviors exhibited a redox-responsive release profile. In detail, about 88.7% and 74.5% TPZ and Pt species were released when incubating polyprodrug@TPZ in a glutathione (GSH, 10 mM) containing solution for 50 h. After 24 h of incubation in a reduction environment, the red solutions in the dialysis bag became colorless and transparent (Fig. 2d). These results revealed that polyprodrug@TPZ might serve as a controllable drug delivery system in vivo, resulting in a reduction of severe side-effects on normal tissues.
Expression of NOX isoforms in polyprodrug-treated A549 cells
NOXs are important sources of ROS.36–39 Previous studies indicate that platinum toxicity is strongly associated with the generation of ROS mediated by NOXs in cancer cells.17,19,40 However, the NOX family comprises seven isoforms that display distinct patterns in different tissues.18,41 The specific binding of NOX-related regulatory subunits and NOX isoforms plays a key role in regulating nicotinamide adenine dinucleotide phosphate (NADPH) activity. For instance, the unique binding partners of NOX2 are p67phox and p47phox through p22phox, whereas the binding partners of NOX1 are NOXA1 (p67phox homolog) and NOXO1 (p47phox homolog).41–44 Therefore, to confirm which NOX isoform can be activated and induced by the polyprodrug nanogels, mRNA expression of these NOX isoforms was examined in the polyprodrug-treated A549 cells. As shown in Fig. 3a, an early transcriptional activation of NOX1 and NOXO1 was comparably detectable at 6 h after incubation with polyprodrug nanogels, whereas NOXA1 and p22phox mRNA levels were not changed. Of note, NOX1 and NOXO1 mRNA levels were increased in a time-dependent manner by the polyprodrug nanogels. Interestingly, NOX2, NOX3 and NOX4 mRNAs were not detected in A549 cells (Fig. S7−S9†). The results indicated that the expression of NOX1 and NOXO1 was upregulated in A549 cells treated with the polyprodrug nanogels. Immunocytochemistry analysis was applied to further verify the cellular localization and expression of NOX1 in A549 cells treated with the polyprodrug nanogels. Neither NOXO1 nor NOX1 was expressed in the control A549 cells (Fig. 3b) that were supplemented with antibodies against NOXO1 (green colour; Alexa Fluor 488-labeled) and NOX1 (red colour; Alexa Fluor 568-labeled). In contrast, both NOXO1 and NOX1 were obviously expressed in the cells after exposure to the polyprodrug nanogels. Next, the mRNA expression of NOX1 was measured in A549 cells treated with the polyprodrug nanogels (20 μM Pt). As expected, a significant increase of NOX1 mRNA level was observed when A549 cells were treated with polyprodrug nanogels for 12 h. However, over 50% NOX1 expression was downregulated by NOX1 siRNA transfected A549 cells (Fig. S12†). The results indicated that the transcriptional activation by polyprodrug nanogels resulted in the increase of NOX activity.Polyprodrug-induced ROS production and O2 depletion via NOX activation
To gain further insight into the mechanisms underlying NOX1-induced ROS production, the intracellular level of ROS was manipulated by 2′,7′-dichlorofluorescein diacetate (DCFH-DA, a ROS-sensitive organic probe).45 Herein, diphenyleniodonium chloride (DPI, a non-specific NOX inhibitor) was used to inhibit NOX1 activity.46 After incubated with 20 μM polyprodrug or polyprodrug@TPZ nanogels for 12 h, the production of intracellular ROS was significantly increased (Fig. 3d and S13†), whereas the intracellular ROS level was reduced after co-incubation with DPI. Upon transfecting the A549 cells with NOX1-specific siRNA constructs, weak green fluorescence was also observed, indicating that NOX1 played a key role in polyprodrug-mediated ROS production. The results implied that ROS generation in A549 cells treated with polyprodrug nanogels was mediated by a NOX pathway. As a balance, intracellular O2 depletion might progress simultaneously with ROS generation in A549 cells treated with polyprodrug nanogels. To demonstrate this hypothesis, oxygen depletion rate was measured by using a dissolved oxygen meter. After co-incubation with polyprodrug or polyprodrug@TPZ nanogels, notably increased oxygen-consuming activity was observed (Fig. 3c). Compared with the control group, oxygen depletion ability in A549 cells was inhibited by co-incubation with DPI or transfection with NOX1 siRNA, demonstrating that the route of O2 depletion was mediated by NOX. These phenomena provide strong evidence indicating that the polyprodrug nanogels might be a good O2 depletion carrier for hypoxia-activated prodrug therapy.In vitro cytotoxicity effects of polyprodrug@TPZ nanogels
Previous reports indicate that NOX-mediated ROS production activates hypoxia-inducible factor-1 (HIF-1).47,48 Hence, we suspect that the exaggerated hypoxia by polyprodrug-induced O2 depletion might further activate TPZ to form highly cytotoxic radicals to destroy nearby macromolecules. To verify this theory, MTT assay was performed. As shown in Fig. 4a, the IC50 value of A549 cells treated with polyprodrug@TPZ was ∼5.0 μg mL−1, whereas the IC50 value of polyprodrug@TPZ was decreased to ∼0.6 μg mL−1 in the hypoxic environment. Once A549 cells were transfected with NOX1 siRNA, the IC50 value of polyprodrug@TPZ was increased to ∼10.5 μg mL−1, attributed to the dysfunction of NOX1 that inhibits ROS production and O2 depletion. The method of Chou and Talalay was applied to evaluate the synergistic effect.49 As shown in Table S2,† the combination index (CI) value was 0.76 in IC50 value of polyprodrug@TPZ, indicating that the Pt-based chemodrug and TPZ were synergistic. To investigate the hypoxia induced by polyprodrug@TPZ, western blot was carried out (Fig. 4b). It could be noticed that expression of HIF-1α in the polyprodrug@TPZ group retained an elevated level at 24 h. After transfection with the NOX1 siRNA, the expression of HIF-1α was inhibited, implying that hypoxia induced by polyprodrug@TPZ could be achieved in vitro.In vivo pharmacokinetics, biodistribution, and immunofluorescence assay of polyprodrug@TPZ nanogels
Encouraged by the potency of polyprodrug@TPZ in vitro, we further assessed its pharmacokinetics by intravenously injecting polyprodrug@TPZ nanogels (2 mg TPZ per kg) into SD rats, while free TPZ was used as the control. Due to the existence of MPC and the nanosized effect,50,51 polyprodrug@TPZ nanogels had a long half-life time (t1/2: ∼9.5 h) (Fig. 5a). In contrast, the half-life time of TPZ was only 14.0 min. The prolonged half-life time explicitly revealed that introduction of zwitterionic moieties can improve the blood circulation time and impede nonspecific adsorption from proteins in blood. To verify whether a long circulation time can enhance antitumor agent accumulation in tumor tissues, live animal imaging was also performed. After intravenously injecting free Cy5.5 or Cy5.5-loaded polyprodrug nanogels into the A549 tumor-bearing mouse, the free Cy5.5 was rapidly cleared out and the fluorescence signal of the tumor tissues was extremely low (Fig. 5b). In contrast, the fluorescence intensity of Cy5.5 gradually increased in the tumor tissues when the Cy5.5-loaded polyprodrug nanogels were used. The most accumulation of polyprodrug nanogels could be observed after 12 h post-injection. A strong fluorescence signal in tumor tissues could be obtained even up to 24 h post-injection, indicating a long retention of the polyprodrug nanogels. Encouraged by the effective hypoxia induced by the polyplatinum(IV) complex-based nanogels in vitro, we next investigated whether polyprodrug@TPZ had the ability to regulate the tumor environment in vivo by immunofluorescence assay. After 24 h post intravenous injection of polyprodrug@TPZ, the A549 cell tumor bearing BALB/c nude mice were scarified and all tumor tissues were fixed and imaged for biochemical analysis. Hypoxic areas, cell nuclei, and blood vessels were stained with antipimonidazole antibody (green colour), DAPI (blue colour), and anti-CD31 (red colour), respectively. After intravenously injecting polyprodrug@TPZ nanogels, green fluorescence increased over time, suggesting polyprodrug@TPZ obviously aggravated the hypoxia in the entire tumor area (Fig. 5c). These results confirmed that the polyprodrug@TPZ nanogels can induce hypoxia in vivo, which may activate toxicity of TPZ for tumor therapy.In vivo anticancer therapy of polyprodrug@TPZ nanogels
Finally, the in vivo anti-tumor efficacy of the hypoxia-activated polyprodrug@TPZ was evaluated on A549 non-small cell lung cancer xenografted mouse models. In detail, each mouse was xenografted on the right back. The A549 xenograft tumor-bearing mice were randomly divided into 5 groups and intravenously injected with saline, TPZ, TPZ + cisplatin, polyprodrug, and polyprodrug@TPZ, respectively, administering once every two days 5 times. The body weights and tumor sizes were measured every two days. Compared with the saline group, the free drug showed negligible effects on inhibiting tumor growth, which exhibited a rapid increase over time (Fig. 6a). In contrast, a slight increase of tumor volume was observed in mice intravenously injected with polyprodrug nanogels, suggesting a moderate therapeutic efficacy of the injected nanoparticles. After loading with TPZ, a significant therapeutic efficacy can be realized by injecting polyprodrug@TPZ. After the treatment, some xenografted tumors had been completely inhibited (Fig. 6c and d). Importantly, the body weight continually decreased in mice treated with TPZ + cisplatin, whereas there were insignificant variations of body weight for mice injected with polyprodrug nanogels (Fig. 6b), implying the negligible systemic toxicity of polyprodrug@TPZ. Lastly, the standard hematoxylin and eosin (H&E) stain was applied to A549 tumor slices obtained by sacrificing mice of different groups after the tumor therapy. As shown in Fig. S15,† most of the tumor section lost its normal morphology for mice treated with polyprodrug and polyprodrug@TPZ simultaneously. To further investigate the level of cell apoptosis in tumor tissue, TUNEL analysis was also performed. Comparatively, apparent green fluorescence could be found in mice injected with polyprodrug@TPZ nanogels, implying the high apoptotic rate of A549 cells as well.Conclusions
In conclusion, we have developed novel polymerized platinum(IV) compound-based nanogels that can not only release cytotoxic Pt(II) species but also promote the hypoxic environment to enhance the cytotoxicity of bioreductive TPZ for a synergistic chemotherapy. When exposed to a cancer microenvironment, the polyprodrug nanogels prepared by copolymerizing the zwitterionic MPC monomer and Pt(IV)-based prodrug monomer could be rapidly reduced to generate cytotoxic platinum(II) drug. More importantly, upregulated expression of NOX1 was induced simultaneously, as confirmed by immunofluorescence assay and western blot, which triggered significant O2 consumption and ROS production in vitro and in vivo. The hypoxia mediated by NOX1 further stimulates the hypoxia-activated prodrug TPZ to generate highly cytotoxic radicals for a synergistic antitumor therapy. With the nanosized architecture and controllable delivery design, the polyprodrug@TPZ nanogels exhibit longer term circulation, more tumor accumulation, and higher therapeutic efficacy than free drug both in vitro and in vivo. This suggests that the therapy based on platinum complex-based chemodrug-exaggerated hypoxia to activate a hypoxia-activated prodrug can serve as a potential method for hypoxic tumor treatment.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2018YFC1106100, 2018YFC1106102), the National Basic Research Program of China (2015CB931801), the National Natural Science Foundation of China (51690151, 21504053, 21661162001), the Program of Shanghai Medical Professionals Across Subject Funds (YG2016MS74), and the China Postdoctoral Science Foundation (2018M640780).Notes and references
- W. R. Wilson and M. P. Hay, Nat. Rev. Cancer, 2011, 11, 393–410 CrossRef CAS PubMed.
- A. L. Harris, Nat. Rev. Cancer, 2002, 2, 38–47 CrossRef CAS PubMed.
- B. Keith and M. C. Simon, Cell, 2007, 129, 465–472 CrossRef CAS PubMed.
- Q. Wang, L. Tian, J. Xu, B. Xia, J. Li, F. Lu, X. Lu, W. Wang, W. Huang and Q. Fan, Chem. Commun., 2018, 54, 10328–10331 RSC.
- Y. Liu, Y. Liu, W. Bu, C. Cheng, C. Zuo, Q. Xiao, Y. Sun, D. Ni, C. Zhang, J. Liu and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 8105–8109 CrossRef CAS PubMed.
- L. Feng, L. Cheng, Z. Dong, D. Tao, T. E. Barnhart, W. Cai, M. Chen and Z. Liu, ACS Nano, 2017, 11, 927–937 CrossRef CAS PubMed.
- C. Qian, P. Feng, J. Yu, Y. Chen, Q. Hu, W. Sun, X. Xiao, X. Hu, A. Bellotti, Q.-D. Shen and Z. Gu, Angew. Chem., Int. Ed., 2017, 56, 2588–2593 CrossRef CAS PubMed.
- D. Guo, S. Xu, N. Wang, H. Jiang, Y. Huang, X. Jin, B. Xue, C. Zhang and X. Zhu, Biomaterials, 2017, 144, 188–198 CrossRef CAS PubMed.
- S. Xu, X. Zhu, C. Zhang, W. Huang, Y. Zhou and D. Yan, Nat. Commun., 2018, 9, 2053 CrossRef PubMed.
- D. Zhang, Z. Cai, N. Liao, S. Lan, M. Wu, H. Sun, Z. Wei, J. Li and X. Liu, Chem. Sci., 2018, 9, 7390–7399 RSC.
- K. Zhang, Y. Zhang, X. Meng, H. Lu, H. Chang, H. Dong and X. Zhang, Biomaterials, 2018, 185, 301–309 CrossRef CAS PubMed.
- L. Shan, W. Fan, W. Wang, W. Tang, Z. Yang, Z. Wang, Y. Liu, Z. Shen, Y. Dai, S. Cheng, O. Jacobson, K. Zhai, J. Hu, Y. Ma, D. O. Kiesewetter, G. Gao and X. Chen, ACS Nano, 2019, 13, 8903–8916 CrossRef CAS PubMed.
- S. Yang, Z. Tang, C. Hu, D. Zhang, N. Shen, H. Yu and X. Chen, Adv. Mater., 2019, 31, 1805955 CrossRef PubMed.
- X. Wang, X. Wang and Z. Guo, Acc. Chem. Res., 2015, 48, 2622–2631 CrossRef CAS PubMed.
- J. J. Wilson and S. J. Lippard, Chem. Rev., 2014, 114, 4470–4495 CrossRef CAS PubMed.
- R. Marullo, E. Werner, N. Degtyareva, B. Moore, G. Altavilla, S. S. Ramalingam and P. W. Doetsch, PLoS One, 2013, 8, e81162 CrossRef PubMed.
- S.-J. Kim, J. Ho Hur, C. Park, H.-J. Kim, G.-S. Oh, J. N. Lee, S.-J. Yoo, S.-K. Choe, H.-S. So, D. J. Lim, S. K. Moon and R. Park, Exp. Mol. Med., 2015, 47, e142 CrossRef CAS PubMed.
- H.-J. Kim, J.-H. Lee, S.-J. Kim, G. S. Oh, H.-D. Moon, K.-B. Kwon, C. Park, B. H. Park, H.-K. Lee, S.-Y. Chung, R. Park and H.-S. So, J. Neurosci., 2010, 30, 3933–3946 CrossRef CAS PubMed.
- Y. Dai, S. Cheng, Z. Wang, R. Zhang, Z. Yang, J. Wang, B. C. Yung, Z. Wang, O. Jacobson, C. Xu, Q. Ni, G. Yu, Z. Zhou and X. Chen, ACS Nano, 2018, 12, 455–463 CrossRef CAS PubMed.
- Y. Nisimoto, B. A. Diebold, D. Cosentino-Gomes and J. D. Lambeth, Biochemistry, 2014, 53, 5111–5120 CrossRef CAS PubMed.
- P. a. Ma, H. Xiao, C. Yu, J. Liu, Z. Cheng, H. Song, X. Zhang, C. Li, J. Wang, Z. Gu and J. Lin, Nano Lett., 2017, 17, 928–937 CrossRef CAS PubMed.
- D. Guo, S. Xu, Y. Huang, H. Jiang, W. Yasen, N. Wang, Y. Su, J. Qian, J. Li, C. Zhang and X. Zhu, Biomaterials, 2018, 177, 67–77 CrossRef CAS PubMed.
- R. S. Smith, Z. Zhang, M. Bouchard, J. Li, H. S. Lapp, G. R. Brotske, D. L. Lucchino, D. Weaver, L. A. Roth, A. Coury, J. Biggerstaff, S. Sukavaneshvar, R. Langer and C. Loose, Sci. Transl. Med., 2012, 4, 153ra132–153ra132 Search PubMed.
- H. Chen, L. Yuan, W. Song, Z. Wu and D. Li, Prog. Polym. Sci., 2008, 33, 1059–1087 CrossRef CAS.
- L. Chen, Y.-a. Yin, Y.-x. Liu, L. Lin and M.-j. Liu, Chin. J. Polym. Sci., 2017, 35, 1181–1193 CrossRef CAS.
- M. Khuphe, N. Ingram and P. D. Thornton, Nanoscale, 2018, 10, 14201–14206 RSC.
- G. Wen, Z. Guo and W. Liu, Nanoscale, 2017, 9, 3338–3366 RSC.
- Q. Zhang, X. Tang, Z.-F. Zhang, R. Velikina, S. Shi and A. D. Le, Clin. Cancer Res., 2007, 13, 4686–4694 CrossRef CAS PubMed.
- H. M. Schuller, Nat. Rev. Cancer, 2009, 9, 195 CrossRef CAS PubMed.
- D. Mei, L. Zhao, B. Chen, X. Zhang, X. Wang, Z. Yu, X. Ni and Q. Zhang, Drug Delivery, 2018, 25, 493–503 CrossRef CAS PubMed.
- L. Han, C. Liu, H. Qi, J. Zhou, J. Wen, D. Wu, D. Xu, M. Qin, J. Ren, Q. Wang, L. Long, Y. Liu, I. Chen, X. Yuan, Y. Lu and C. Kang, Adv. Mater., 2019, 31, 1805697 CrossRef PubMed.
- D. Wu, M. Qin, D. Xu, L. Wang, C. Liu, J. Ren, G. Zhou, C. Chen, F. Yang, Y. Li, Y. Zhao, R. Huang, S. Pourtaheri, C. Kang, M. Kamata, I. S. Y. Chen, Z. He, J. Wen, W. Chen and Y. Lu, Adv. Mater., 2019, 31, 1807557 CrossRef PubMed.
- V. Kouranos, G. Dimopoulos, A. Vassias and K. N. Syrigos, Cancer Lett., 2011, 313, 9–14 CrossRef CAS PubMed.
- N. A. G. dos Santos, M. A. C. Rodrigues, N. M. Martins and A. C. dos Santos, Arch. Toxicol., 2012, 86, 1233–1250 CrossRef PubMed.
- W. Wan, M. Biyikal, R. Wagner, B. Sellergren and K. Rurack, Angew. Chem., Int. Ed., 2013, 52, 7023–7027 CrossRef CAS PubMed.
- J. Foreman, V. Demidchik, J. H. F. Bothwell, P. Mylona, H. Miedema, M. A. Torres, P. Linstead, S. Costa, C. Brownlee, J. D. G. Jones, J. M. Davies and L. Dolan, Nature, 2003, 422, 442–446 CrossRef CAS PubMed.
- M. Ushio-Fukai and Y. Nakamura, Cancer Lett., 2008, 266, 37–52 CrossRef CAS PubMed.
- H.-M. Gao, H. Zhou and J.-S. Hong, Trends Pharmacol. Sci., 2012, 33, 295–303 CrossRef PubMed.
- K. Block and Y. Gorin, Nat. Rev. Cancer, 2012, 12, 627 CrossRef CAS PubMed.
- T. Kaur, D. Mukherjea, K. Sheehan, S. Jajoo, L. P. Rybak and V. Ramkumar, Cell Death Dis., 2011, 2, e180 CrossRef CAS PubMed.
- S. Altenhöfer, K. A. Radermacher, P. W. M. Kleikers, K. Wingler and H. H. Schmidt, Antioxid. Redox Signaling, 2014, 23, 406–427 CrossRef PubMed.
- S.-E. Cheng, I. T. Lee, C.-C. Lin, W.-L. Wu, L.-D. Hsiao and C.-M. Yang, PLoS One, 2013, 8, e54125 CrossRef CAS PubMed.
- G. Cheng and J. D. Lambeth, J. Biol. Chem., 2004, 279, 4737–4742 CrossRef CAS PubMed.
- I.-T. Lee, S.-W. Wang, C.-W. Lee, C.-C. Chang, C.-C. Lin, S.-F. Luo and C.-M. Yang, J. Immunol., 2008, 181, 5098–5110 CrossRef CAS PubMed.
- C. P. LeBel, H. Ischiropoulos and S. C. Bondy, Chem. Res. Toxicol., 1992, 5, 227–231 Search PubMed.
- V. V. Sumbayev, FEBS Lett., 2008, 582, 319–326 CrossRef CAS PubMed.
- E. J. Moon, P. Sonveaux, P. E. Porporato, P. Danhier, B. Gallez, I. Batinic-Haberle, Y.-C. Nien, T. Schroeder and M. W. Dewhirst, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20477–20482 CrossRef CAS PubMed.
- G. Yuan, J. Nanduri, S. Khan, G. L. Semenza and N. R. Prabhakar, J. Cell. Physiol., 2008, 217, 674–685 CrossRef CAS PubMed.
- T.-C. Chou, Cancer Res., 2010, 70, 440–446 CrossRef CAS PubMed.
- L. Zhang, Y. Liu, G. Liu, D. Xu, S. Liang, X. Zhu, Y. Lu and H. Wang, Nano Res., 2016, 9, 2424–2432 CrossRef CAS.
- S. Liang, Y. Liu, X. Jin, G. Liu, J. Wen, L. Zhang, J. Li, X. Yuan, I. S. Y. Chen, W. Chen, H. Wang, L. Shi, X. Zhu and Y. Lu, Nano Res., 2016, 9, 1022–1031 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9bm01640f |
| This journal is © The Royal Society of Chemistry 2020 |