Glucosamine sulphate-loaded distearoyl phosphocholine liposomes for osteoarthritis treatment: combination of sustained drug release and improved lubrication†
Abstract
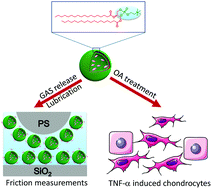
Osteoarthritis (OA) is a chronic joint disease resulting from joint inflammation and damage. In this study, we employed a boundary lubricant known as a 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) liposome for loading of an anti-inflammatory drug D-glucosamine sulphate (GAS) to construct a treatment strategy allowing for sustained anti-inflammation and reduced damage. This kind of drug-loaded nanocarrier integrates the anti-inflammatory effect of the GAS and the lubrication ability of DSPC liposomes without the involvement of complex synthesis processes leading to easier popularization. Our experimental results indicated that the GAS-loaded DSPC liposomes could release GAS in a sustained manner while providing good lubrication in pure water (H2O) and phosphate buffered saline (PBS). Moreover, the GAS-loaded DSPC liposomes prepared at a 2 : 8 molar ratio in PBS exhibited a greater entrapment efficiency, lower GAS release rate and smaller friction coefficient as compared to those prepared in H2O. The superiority of the drug release and lubrication ability achieved with the GAS-loaded DSPC liposomes in PBS were elucidated on the basis of salt-induced enhancement in liposomal stability and hydration lubrication by the hydrated salt ions. Such GAS release accelerated the viability and proliferation of primary mouse chondrocytes while also providing the anti-inflammatory and chondroprotective potential for tumor necrosis factor (TNF-α) induced chondrocyte degeneration through the down-regulation of pro-inflammatory cytokines, pain related gene and catabolic proteases, as well as the up-regulation of anabolic components. We envision that the GAS-loaded DSPC liposomes could represent a promising new strategy for clinical treatment of OA in the future.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...