Open Access Article
Open Access ArticleCurrent concepts in nanostructured contrast media development for in vivo photoacoustic imaging
Mirko
Maturi
 ,
Erica
Locatelli
,
Erica
Locatelli
 ,
Ilaria
Monaco
and
Mauro
Comes Franchini
,
Ilaria
Monaco
and
Mauro
Comes Franchini
 *
*
Department of Industrial Chemistry “Toso Montanari”, University of Bologna, Viale Risorgimento 4, 40136 Bologna, Italy. E-mail: mauro.comesfranchini@unibo.it; Fax: +051-2093654; Tel: +051-2093626
First published on 22nd March 2019
Abstract
Photoacoustic (PA) imaging is indeed one of the most promising bioimaging techniques for theranostics applications in humans, allowing for the visualization of blood vessels and melanomas with high spatial resolution. However, in order to overcome the endogenous contrast arising from interfering endogenous species such as haemoglobin and melanin, specific contrast agents need to be developed, allowing PAI to successfully identify targeted contrast in the range of wavelengths in which interference from the biomatrix is minimized. This has been first performed by small molecule dyes, which, however, suffer from some important limitations such as low hydrophilicity and short circulation times. For this reason, scientific research has recently directed its efforts towards the development of nanostructured contrast agents capable of providing efficient PA contrast at low concentrations with low toxicity and high biocompatibility. The principal nanostructures are based on (1) metal and semiconducting nanoparticles, amongst which variously shaped nano-gold plays the main role, (2) carbon nanomaterials, such as carbon nanotubes and graphene, and (3) conjugated polymer nanoparticles. In this review, the principal characteristics of this class of materials are reported and greater focus is directed towards in vivo studies. A detailed analysis is performed on various physical–chemical parameters that define the PA response of reported contrast agents, like absorption coefficients and photoacoustic efficiencies. By comparing the experimental data, this review provides a comprehensive tool for the evaluation of new nanostructured contrast agents for PA imaging.
Introduction
Photoacoustic (PA) imaging is an emerging technique based on the illumination of a specimen with NIR nanosecond-pulsed laser light and the absorption of photons by chromophores in the sample results in the generation of acoustic waves, which are then detected and processed to form an image. Thanks to acoustic detection, it is often possible to couple PA imaging to ultrasound (US) echographic systems: ultrasounds are directed to the sample together with the laser, scattered and diffused in the tissues and detected back by a separate US detector.1 Although PA and US image formation and the factors that affect spatial resolution are mostly the same, the contrasts that generate the images are essentially different. A US image describes the acoustic impedance mismatch between different tissues: US contrast, therefore, depends on the morphological features of the tissues, while PA represents the initial pressure distribution produced by the absorption of a pulse of laser light. The capability to perform photoacoustic detection on non-transparent samples provides the possibility of exploiting it for spectral characterization of biologic tissues, for which traditional optical spectroscopic tools cannot be generally applied. The field of optoacoustics has rapidly grown in terms of development of instrumentation, image processing algorithms, in vivo application of the technique in clinical medicine and basic biological research.2 In photoacoustic (PA) imaging, ultrasound waves are generated by irradiating tissues with modulated or pulsed laser light. For applications in biology and biomedicine, optical wavelengths in the near-infrared (NIR, 700–1500 nm) are applied due to the high transparency of tissues in this range, achieving penetration depths up to several centimeters. In this region of the electromagnetic spectrum are located the two so-called biological windows, in which the absorption from biological tissues is minimized (or transparency is maximized). Recently, PA imaging has emerged in biomedicine thanks to its easy translation in human studies and its remarkable features compared to most other imaging techniques. PA imaging allows for extremely high spatial resolution which is only limited by the technological implementation of the ultrasonic transducer. With respect to traditional optical imaging (fluorescence or phosphorescence), PA imaging employs a much longer irradiation wavelength, with a consequent increase in penetration depth and in safety associated with laser employment. Moreover, the merging of light- and sound-based approaches allows for the evaluation of molecular spectral features of the analysed specimen together with its morphological details, to which ultrasounds are mostly sensitive. Although organic tissues can generate strong PA signals, the employment of specific contrast agents is desirable to retrieve detailed information, and most of the research activity has focused on this aspect.Within the field of photoacoustics, the currently most exciting challenges are represented by the design and development of nanostructured contrast agents associated with increasingly higher contrast-to-noise ratios, little or no toxicity, and rapid clearance from the circulatory vessels. In addition, by moving the absorption of contrast agents to higher wavelengths, it will be possible to achieve much greater penetration depths and lower interference from endogenous chromophores. With this approach, PA is predicted to become an extensively versatile tool for diagnostics in medicine. With the present work, we aim to review the fundamental applications of nanostructured contrast agents for PA imaging of biological systems in vivo, with a particular focus on the peculiar properties that allow each of the different nanostructures to generate PA signals. First, the main physical–chemical properties of nanostructures will be examined, and then their PA performances will be compared and the most remarkable results shown.
Endogenous contrast
Standard implementations for PA imaging require laser-emitting light in the near-infrared window of the electromagnetic spectrum (λ = 800–2000 nm) since shorter wavelengths would be scattered and absorbed to a greater extent. Since NIR light can achieve high penetration depth in biological tissues of the order of several centimeters, part of the incoming photons will be absorbed by chromophores naturally present in the tissues such as lipids, water, collage, melanin and hemoglobin in its two forms, oxygenated (Hb) and deoxygenated (HbO2); these are referred to as endogenous chromophores (Fig. 1a). Hemoglobin is an iron-based metalloprotein responsible for the delivery of molecular oxygen throughout the body: it undergoes structural and electronic modifications upon O2 binding, which results in a modification of its absorption spectrum. This allows measuring the total hemoglobin concentration and oxygen saturation by multispectral PA imaging, which provides helpful information for the study of tumor angiogenesis.5–9 It is also worth mentioning the absorption behavior of lipids at long wavelengths (930 and 1210 nm) related to the second overtone of the C–H stretching, which are abundant in fatty acid chains.10,11 Contrast can arise from water if excited at 975 nm, whereas melanin, thanks to its intense absorption throughout the biological window, allows for the detection and characterization of primary melanoma and metastatic melanoma cells by PA imaging.12 However, melanin strongly influences the penetration depth achievable with PA imaging, drastically limiting the performances of PA imaging in melanin-rich tissues. However, this can be exploited to employ melanin-based nanomaterials as exogenous contrast agents, after the required chemical modification.13 Anyway, intrinsic chromophores only give access to a limited number of applications in biological processes. In PA imaging for biomedical applications, these absorptions represent the background that must be overcome by efficient contrast agent engineering capable of highlighting specific features of the investigated biological system. The absorption profiles of endogenous chromophores generate two distinct biological windows in the NIR between 650 and 950 nm and the second between 1000 and 1350 nm, separated by the strong absorption from lipids at 950–1000 nm (Fig. 1b). Even though endogenous contrast can be significantly relevant for particular tissues or for particular excitation wavelengths, several multivariate analysis tools have been developed for the resolution of the spectral mixtures in PAI. For example, the implementation of multivariate curve resolution (MCR) algorithms for the decomposition of multispectral PA images in distribution maps of the single components of the endogenous PA contrast has been recently reported.14,15 The multivariate separation of endogenous and exogenous contrast in PA imaging is yet to be explored, but this could make contrast-based PA imaging an important non-invasive diagnostic tool in the near future.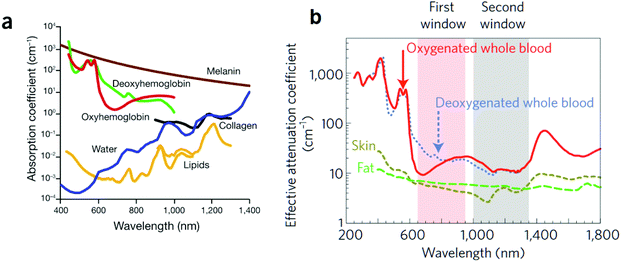 | ||
| Fig. 1 Endogenous chromophores. (a) Absorption spectra of the main endogenous chromophores. At long wavelengths, the contribution of oxy- and deoxy-hemoglobin is negligible but the interference of water and fat is increasing. Melanin covers the whole spectral range, strongly reducing the optical penetration depth in melanin-rich regions. (b) Attenuation coefficients for oxygenated and deoxygenated blood, fat and skin, with the two biological windows highlighted. Adapted from ref. 3 and 4. | ||
Contrast media for PA imaging in vivo
In 2017 the global contrast agent market size was valued at USD 4.89 billion and is anticipated to constantly grow (Fig. 2). Leading categories in the market are barium-based, iodinated, gadolinium-based, and microbubble contrast media: this is attributable to the preferential exploitation in the clinical approach of X-ray and CT procedures, as well as MRI procedures. However, these materials are currently under regulatory scrutiny, which is expected to prove detrimental for the market until the arrival of safer alternative agents. From the application point of view, neurological disorders account for the largest share, followed by cardiovascular disorders and cancer. High applicability of imaging modalities and accessibility to launched products for diagnosis of cancer or other diseases are strongly required from the actual market.16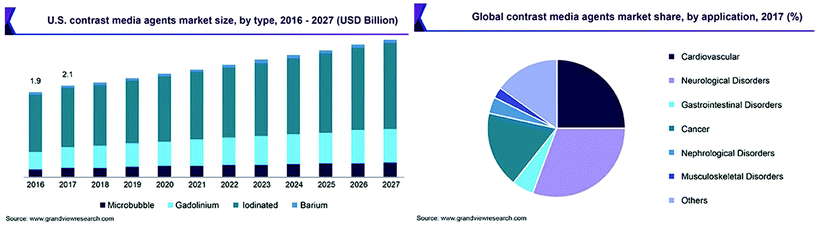 | ||
| Fig. 2 Left: market size trends expected for contrast agents. Right: application of contrast agents in 2017. Adapted from ref. 16. | ||
As has already been discussed, the need for exogenous contrasts arises from the diffusion and non-specificity of endogenous PA-responding macromolecules. Although endogenous chromophores can provide useful information about blood flow, oxygen saturation and lipid content, they do not make PA suitable for the detection of specific diseases in vivo. Contrariwise, smartly-engineered nanostructured contrast media can be precisely designed for each particular application in diagnostics and, sometimes, as theranostic agents. In the present section, the most outstanding classes of exogenous contrast media for PA imaging will be presented together with their main physical–chemical properties. Firstly, molecular contrast performed by small molecule dyes will be briefly discussed, and then our focus will be mainly directed towards nanostructured contrast agents which are currently available in the literature.
Small molecule dyes
Small molecule dyes (SMDs) are polyconjugated organic chromophores; for application as PA contrast agents in deep tissues, absorption in the NIR is required. The optimal dye shows extended conjugation, which leads to the contraction of the HOMO–LUMO gap energies to energies corresponding to the near-infrared region of the electromagnetic spectrum: upon the absorption of NIR radiation, an electron of the dye is promoted from its HOMO to its LUMO, and relaxation of the excited states occurs via non-radiative processes (heat-wave), leading to anisotropic photoacoustic emission. Moreover, the fluorescence quantum yield of the dye should be as low as possible to maximize photoacoustic efficiency and contrast.17,18 The brightest future for this class of PA contrast media is expected to be experienced by the FDA-approved indocyanine green (ICG) (Fig. 3a). It has a strong and characteristic concentration-dependent absorption band at around 800 nm in diluted solutions (Fig. 3d); it is water soluble and biocompatible and shows intense fluorescence emission in the NIR (around 850 nm) with low radiative quantum yield (2.7%).19,20 Its improved photoacoustic performances have been attributed to the high lipophilicity of the molecule, which causes attraction between molecules by van der Waals interactions leading to extensive non-radiative relaxation of the excited states generated by laser light absorption.21 The potential application as PA contrast agents of ICG, existing in the free molecular form or conjugated to specific targeting agents, has been widely investigated in recent years, showing high plasma protein binding and promising results as blood flow monitoring agents and providing good tumor contrast.22–26 However, ICG suffers from some important drawbacks shared with most SMDs: poor aqueous stability, concentration-induced aggregation and quick clearance (around 2–4 min) from the body. In parallel, other classes of dyes have been explored as contrast agents for molecular PA imaging; amongst these, methylene blue (MB) has rapidly emerged thanks to its well-known biocompatibility and low photobleaching, together with its unique optical properties due to its strong absorption band centered at 664 nm (Fig. 3e), which may blue-shift with increasing concentration due to oligomerization equilibria.27 The features of MB (Fig. 3b) have been recently exploited for several in vivo studies displaying good photoacoustic contrast in vivo after subcutaneous injection in mice for sentinel lymph node detection and photoacoustic imaging of the bladder, but due to the relatively shorter wavelength of the absorbed radiation, poor penetration depth can be achieved.28–30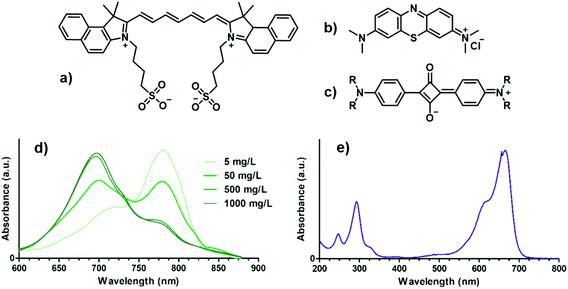 | ||
| Fig. 3 Chemical structures of (a) ICG, (b) MB and (c) squaraine dyes, and UV-VIS spectra of (d) ICG at different concentrations and (e) MB. | ||
Another representative class of SMD are squaraine dyes, characterized by an electron-deficient cyclobutene ring conjugated with two electron deficient groups (Fig. 3c); their optical properties can be tuned to the NIR region by adjusting the electron-donating strength of the conjugated groups.31 Even though they show bright photoacoustic contrast due to their longer absorption wavelength and lower fluorescence quantum yield, their applications are limited by their intrinsic lipophilicity and chemical reactivity.
Since the most important requirement for an organic dye to be employed in PA is the NIR absorption, several different classes of small molecule dyes have been developed and studied in addition to the abovementioned compounds. Recently, appositely formulated naphthalocyanine dyes have been exploited as PA signaling compounds, aiming to image in the NIR with increasing sensitivity.32,33 In addition, other dyes such as Prussian blue, Coomassie blue, BODIPYs (4,4-difluoro-4-bora-3a,4a-diaza-s-indacenes), porphyrines and cyanines have been explored for the purpose, showing good contrasts and sensing behaviour in vivo.34,35,36–43,44 However, the development of new small molecule dyes as contrast agents for preclinical PA imaging is strongly limited by their molecular properties: the high conjugation degree required for NIR absorption drastically decreases the water-dispersibility properties of the dyes, leading to low stability in aqueous media and concentration-dependent optical properties. Moreover, no SMD capable of interacting with light waves longer than 800 nm has been reported, and their optical absorption coefficient is still quite low (<5000 M−1 cm−1) compared to most nanostructured photoabsorbers. For these reasons, several methods have been developed recently for coupling ICG, MB and other dyes to a different set of nanostructures, like the encapsulation of dyes in biodegradable polymeric matrices such as PLA, PLGA and PLC, which can drastically increase their circulation lifetimes and their dispersibility in aqueous environments.45,46
While the encapsulation of dyes into polymeric micelles or liposomes has ensured the overcoming of some of their drawbacks, their coupling to metallic nanostructures (magnetic or semiconducting) has allowed for increased stability and imaging in multiple modalities.47–49 The formulation of dye-conjugated nanosystems also allows for chemical functionalization with specific targeting moieties leading to increased specific local contrast.50–57
PA-responsive nanostructures
Photoacoustic-responsive nanostructures are composite nanomaterials capable of efficiently interacting with tissue-penetrating pulsed near-infrared light and to transform it into high-frequency ultrasound waves that can be detected by piezoelectric transducers to generate PA images. Their characteristics must include: (1) small size (<200 nm), allowing for good dispersion and active and passive tumor targeting;58,59 (2) good biocompatibility and minimum cytotoxicity;60 (3) high molar absorption coefficient in the NIR; (4) high photoacoustic efficiency and low fluorescence quantum yield; (5) low photobleaching.61,62 In addition, by smart coupling of the photoactive core with other agents with specific activities, such as radiolabelled compounds, paramagnetic structures or fluorescence probes, multiple-modality imaging (PA-PET, PA-MRI, etc.) is allowed.63 The main classes of nanostructures used as contrast agents for PA imaging are:• Metal and semiconducting nanoparticles, whose optical properties are due to their metallic core (surface plasmon resonance or exciton absorption);
• Carbon nanomaterials, in which peculiar properties of nanostructured carbon allotropes are exploited to generate photoacoustic waves;
• Polymeric nanoparticles, composed of polyconjugated polymers or polymers containing photoactive groups such as porphyrin derivatives (HOMO–LUMO absorption).
Metal and semiconducting nanoparticles
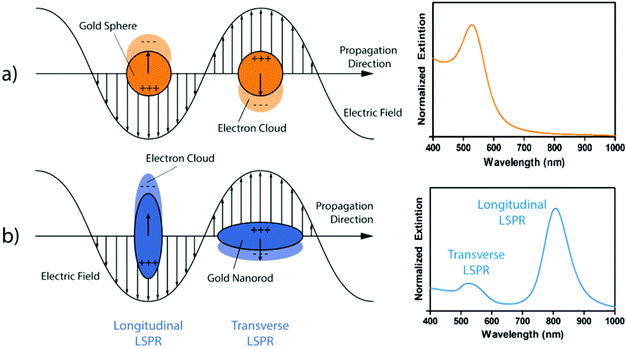
In the field of nanomedicine, the role of transition metal nanoparticles (MNPs) has rapidly grown due to the characteristic properties that their compounds show at the nanoscale.64 The optical properties of MNPs can arise from localized surface plasmon resonance (LSPR) in noble metal nanoparticles or from the formation of an exciton, an electron–hole pair, in semiconducting nanostructures. Also, their surface chemistry is relatively easy and well-known; therefore, it could be exploited for chemical functionalization with biocompatibilizing or targeting agents that need to be specifically developed for each application. The optical properties of MNPs mostly depend on their size and shape; thus, fine-tuning the parameters that determine the evolution of the morphology of the nanoparticle in the synthesis steps is a key factor for the obtainment of the desired properties in the final nanoconstructs. For this reason, MNPs show finely tunable absorption behavior in the NIR region of the electromagnetic spectrum, and experimental evidence reports much higher molar extinction coefficients compared to NIR dyes. Finally, their high thermal conductivity allows for improved conversion of the energy of the absorbed radiation into thermal waves.Besides, due to their larger size, they can accumulate via the enhanced permeability and retention (EPR) effect, allowing for passive tumor targeting.88–91 However, their high surface-to-volume ratio makes them poorly photostable, and they tend to reduce their aspect ratio upon absorption, generating the need for a shell of dielectric material to improve the photostability of the structure.92–95
The optical behavior of nanoshells has been recently described, in which a continuous or semi-continuous layer of gold is deposited on a nanoparticle of a dielectric material.96,97 The nanoshell thickness and radius can be tuned to make it capable of absorbing light in the NIR with extremely high extinction coefficients and improved photostability, allowing for PA contrast at low concentrations.98 Also, the dielectric core material can be chosen to provide the nanoconstruct with additional features such as piezoelectricity and paramagnetism, allowing for applications in photothermal therapy and dual-modality imaging.99,100 In 2005, Millstone et al. first reported the synthesis of small triangular gold nanoprisms which exhibit strong in-plane quadrupole plasmon resonance, resulting in an additional LSPR band at long wavelengths (1200–1400 nm) exploitable for applications in PA imaging.101 Later, the same authors reported the implementation of their synthesis technique, which has then allowed for fine-tuning of the prism edge length and therefore of the quadrupolar LSPR band position.102 The electric field enhancement at the sharp edges of the nanoprisms leads to higher molar extinction coefficients compared to most other gold nanostructures, allowing for the PA imaging of living tissues with strong contrast signals.103–105
Gold nanocages show similar optical properties and can be used for drug or dye entrapment and controlled delivery, making these systems highly advantageous for molecular photoacoustic imaging.106–108 They are generated by the galvanic displacement of Ag atoms on the surface of silver nanoparticles of various shapes in the presence of gold precursor salts, leading to hollow gold nanostructures with tunable optical properties in the NIR, suitable for use as PA contrast agent in vivo.109–111 Moreover, gold nanostars have been recently reported to show characteristic absorption, which can be tuned in the NIR by controlling the size of the nanoparticle and the sharpness of its protuberances.112,113 Nanoscale gold multipods, which are highly branched gold nanostructures similar to gold nanostars, have shown good optical properties in the near-infrared but little tunability.114,115 Therefore, their applications as contrast agents for PA imaging are promising but still quite limited. Fig. 5 presents characteristic TEM images for different morphologies of gold nanostructures from the literature. Extensive work for the manipulation of gold at the nanoscale has recently led to the development of increasingly complex architectures with exotic shapes, which exploit sharp edges to red-shift their SPR absorption up to the NIR.116,117
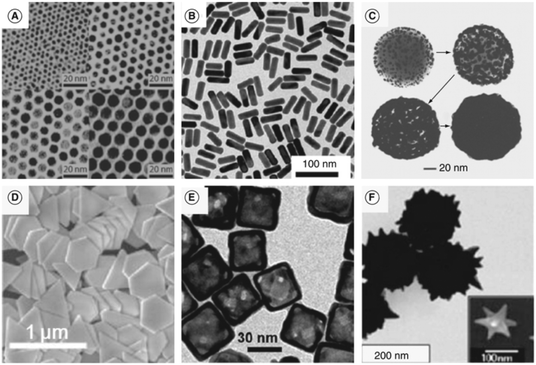 | ||
| Fig. 5 Transmission electron micrograph of (a) gold nanoparticles, (b) gold nanorods, (c) gold nanoshells, (d) gold nanoprisms, (e) gold nanocages and (f) gold nanostars. Adapted from ref. 67. | ||
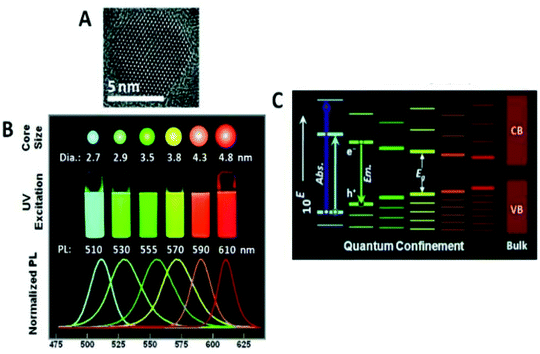 | ||
| Fig. 6 (a) TEM images of CdSe/ZnS QDs; (b) photograph of QDs of different diameters exposed to UV light (365 nm) and their corresponding emission spectrum; (c) representation of the exciton energy levels as a function of the size of the QD. Adapted from ref. 120. | ||
Carbon nanomaterials
Carbon nanomaterials are classified based on their dimensionality, i.e. the number of spatial dimensions in which the nanostructure develops in space:• 0-dimensional (0D): fullerene, carbon dots and nanodiamonds
• 1-dimensional (1D): carbon nanotubes
• 2-dimensional (2D): graphene
As is true for most nanostructured materials, the optical properties of carbon-based nanostructures are strictly dependent on their shape and size. Carbon nanomaterials are generally black, owing to a broad absorption in all the visible and NIR, but the phenomena that generate absorption are different in each case.135 Fullerenes (0D) behave more as molecules than as nanostructures since their photophysical behavior in the visible is related to the HOMO–LUMO transition of one electron upon the absorption of a photon with the right energy. Since they have poor fluorescence properties, they are good candidates for the generation of high-frequency acoustic waves.136
However, their photostability is strongly limited: upon the absorption of NIR laser light, functionalized fullerenes generate acoustic shock waves detectable by PA imaging, which disrupt the spherical structure of the molecules and their optical behavior.137 On the other hand, carbon nanotubes (1D) owe their absorption properties to their semiconducting band gap, which may vary depending on the nanotube diameter and the chiral wrapping angle which describes how it has been constructed by a graphene sheet.138 They also display fluorescence if isolated in micelles, but their photoacoustic response is stable enough to ensure a good photoacoustic response in vivo.139–141 Moreover, graphene (2D) has been reported to be a gapless semiconductor; thus it would show weak absorption features in the NIR. However, due to the abundance of surface heterogeneities, graphene oxide (GO) shows strong absorption and fluorescence in the NIR (Fig. 7).142,143 The exact origins of this behavior is still under debate, but it is believed to be related to the electronic transition at the boundaries between GO and non-oxidized sp2 graphene domains.144,145 Due to their promising optical features and their easy surface functionalization and biocompatibilization, GO and graphene-based nanomaterials have widely shown their potential as contrast media for PA imaging, allowing for efficient imaging of cancer in multiple modalities.146–148 Eventually, nanodiamonds are carbon nanoparticles in which all the atoms are sp3 carbons and no graphitic-carbon is present. Their optical features include strong absorption and weak fluorescence, which arise from local defects in their microcrystalline structure generally induced by other elements like nitrogen.149 On the whole, carbon nanomaterials have shown remarkable results but their absorption in the NIR is relatively broad, thus presenting an important challenge for spectral unmixing and distinction of the contrast from the background; this can be limited by contrast enhancement from NIR dyes attached to the carbon backbone.150–153 Still great effort needs to be directed towards the comprehension of the overall photophysical behavior of carbon nanomaterials: their limited extinction coefficients and low biocompatibility make them only superficially explored contrast agents for in vivo photoacoustic imaging.
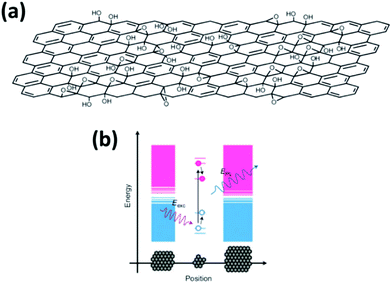 | ||
| Fig. 7 (a) Proposed 3D structure of GO and (b) proposed energy band diagram of GO: larger band gaps are associated with smaller aromatic domain size. Adapted from ref. 143. | ||
Polymeric nanostructures
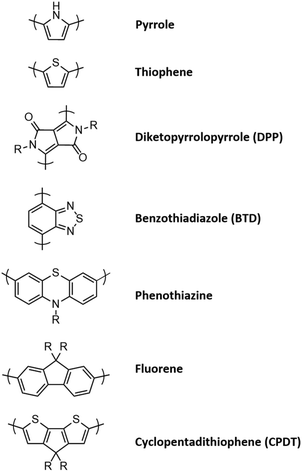
Polymeric nanoparticles (PNPs) have found application in drug delivery due to their high biocompatibility and their ability to encapsulate and protect in their interiors drugs, dyes, proteins, etc.154 In photoacoustic imaging, the most popular polymeric structures contain either conjugated polymers (CPs) or porphyrin-related units capable of directly interacting with NIR light thanks to their elevated π-conjugation spread throughout the whole polymer chains.155 CPs are often referred to as semiconducting polymers (SPs), since their incredibly high conjugation degrees cause the formation of band structures and a massive shrinking in the gap between the π and π* bands, which resembles the features of most inorganic semiconductors.156 By means of microemulsion or nanoprecipitation, CPs are able to self-assemble into nanostructures which are stable and well dispersible in water, known as conjugated polymer nanoparticles (CPNs) or semiconducting polymer nanoparticles (SPNs). Although their extinction coefficients are still lower than the ones of AuNPs and their absorption peak is broad, their high photostability and chemical versatility allow for both specific targeting and attachment of therapeutic moieties. CPs are macromolecules with a conjugated π-system spread throughout the polymeric backbone, and the polymers are densely packed to form nanoparticles with much stronger extinction coefficient and higher photostability compared with small molecule dyes. Optical properties, strictly connected to the polymeric core, can be tuned via modification of the polymeric backbone, combination of different polymers and control over aggregation and surface functionalization.157–160 Because this class of contrast agents is still relatively young, detailed biocompatibility studies aimed at showing their potential for preclinical photoacoustic imaging still need to be conducted.161–167 Principally, semiconducting polymers employed for PA imaging are composed of diketopyrrolopyrrole, phenothiazine, cyclopentadithiophene, fluorene, pyrrole or thiophene monomeric units, allowing for the structural rigidity required for efficient π conjugation (Fig. 8).168–171 Finally, non-absorbing polymeric micelles can be employed for the encapsulation of PA active species such as lipophilic NIR dyes and complex structures, like perylene diimide derivatives.172–178Essential parameters
In the process of PA contrast agent development, some key parameters should necessarily be taken into account to allow for clear comparison amongst different nanostructures.• One of the most important parameters is ε or σ, the molar absorption coefficient or absorption cross section. The first one is the most often reported and it represents the portion of absorbed photons with a specific wavelength in a solution with unitary concentration, and it can be directly used to compare different contrast agents; for example, gold nanostructures display ε between 109 cm−1 M−1 and 1011 cm−1 M−1 but most small molecule dyes have ε ≈ 105 cm−1 M−1 and for SWNTs ε ≈ 106 cm−1 M−1.
• A second important parameter is η, the photoacoustic conversion efficiency, which represents the fraction of photons effectively converted into thermal energy. It is not reported often in the literature, but it completes the information given by ε since only photons which are efficiently absorbed and converted can give rise to PA signals. As reported in Table 1, some reference values for η are 23% for GNRs, 41% for Sb NRs and 48% for TiN nanoparticles.
| Targeting/coating | Size a (nm) | Absorption (nm) | PA excitation (nm) | ε or σb | η | PA enh. d | Injected concentration | A/M e | Toxicityf | Application | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Determined by TEM or DLS. It may refer to the measured hydrodynamic diameter. b Measured molar extinction coefficient (ε) or absorption cross-section (σ). c Photoacoustic efficiency. d Enhancement of the PA signal due to the contrast agent compared to the background or control sample. e Activatable (A) or multimodal (M) probes. f Collects the evaluations on particle toxicity, if evaluated by the authors. | ||||||||||||
| Gold nanoparticles | Lipoic acid-derived citraconic anhydride | 10 | 650 | 680 | 107–109 cm−1 M−1236 | — | 5.7× | 30 nM | A | — | Tumor contrast | 76 |
| Poly-di(carboxylatophenoxy) phosphazene (PCPP) | 200 | >650 | 700 | — | — | 75 μg Au | M | Non-significant | Tissue contrast | 74 | ||
| Diazirine | 49 | 700–900 | 405 and 808 | 78.8% | 4× | 2 mg mL−1 | A | None up to 200 μg mL−1 | Tumor theranostics | 186 | ||
| PEO-b-PCL | 26 | 800 | 808 | 37% | 10× | 400 μg mL−1 | A | Photothermal | 75 | |||
| Ag shell | 84 | 756 | 750 | — | 32× | 15 mg kg−1 | M | — | Vascular imaging | 116 | ||
| SiO2-Gd3+ | 120 | 550 | 532 | — | 73% | 50–1100 pM | M | — | Triple-modality imaging | 185 | ||
| Gold nanocages | PEG | 50 | 825 | 764–824 | 0.036 μm2 | — | 80% | — | — | — | Cerebral cortex imaging | 106 |
| 90 | 680–1000 | 700 | — | — | 5.5× | 0.6 pmol | — | — | Tissue contrast | 192 | ||
| PVP | 50 | 750 | 735 | — | — | 3.5× | 2 nM | — | — | Sentinel lymph node imaging | 111 | |
| PEG-MSH melanocite stimulating hormone | 46 | 778 | 570 and 778 | 1.7 × 10−14 m2 | — | 36% | 10 nM | — | — | Tumor contrast | 194 | |
| Gold nanoprism | PEG-RGD | 110 | 980 and 530 | 710 | — | — | — | 1 mg mL−1 | M | None up to 200 μg mL−1 | Tumor theranostics | 195 |
| PEG-glucose | 94 | 845 | — | — | — | — | — | Photothermal | 196 | |||
| PEG | 110 | 796–911 | 800 | — | — | — | 200 μg mL−1 | — | Non-significant | 105 | ||
| 120 | 830 and 530 | 710 | — | — | — | 744 μg mL−1 | — | Tumor contrast | 103 | |||
| SiO2 | 120 | — | 1064 | — | — | — | — | — | Sentinel lymph node imaging | 104 | ||
| Gold nanorods | PEG | 40 × 12 | 756 | — | 109–1010 cm−1 M−1237 | — | 2.3× | 5.2 nM | M | Non-significant | Tumor contrast | 187 |
| Gd2O2S | 70 × 40 | 820 | — | 22.6% | 3× | 50 μg mL−1 | M | Tumor theranostics | 188 | |||
| Poly(N-isopropylacrylamide-co-methacrylic acid) (PNIPAAmMA) | 60 × 10 | 858 | 800 | — | 2× | 5 mg kg−1 | M | Photothermal | 85 | |||
| Reduced graphene-oxide | 110 | 750 | 700 and 800 | — | 40× | 2.5 nM | — | — | Tumor contrast | 231 | ||
| Melanin | 150 | 600–700 | 764 | — | — | 12 mg mL−1 | — | None up to 2.75 mg mL−1 | Tissue contrast | 77 | ||
| PEI-microRNA | 100 | 815 nm | — | — | — | 0.8 mg mL−1 | M | Photothermal | Tumor theranostics | 190 | ||
| SiO2 | 70 × 30 | 729 nm | 720–740 | — | — | 30 pM | — | — | Stem cells tracking | 93 | ||
| PEG-Alkyne in PLGA-b-PEG | 375 | 680 nm | 690 | — | 10× | 110 μM | M | Hemocompatible | Triple-modality imaging | 234 | ||
| Gold nanoshells | PLGA-b-PEG-folic acid | 165 | 850–900 | 860 | — | — | — | 0.7 mg kg−1 | M | Non-significant | Tumor contrast | 191 |
| PEG-antibody | 40 | 710 | 710 | — | — | — | — | — | 98 | |||
| Gold nanostars | PEG-antibody | 12 | 635 | 720 | — | — | 4.7× | 0.867 mg Au mL−1 | — | Photothermal | Tumor theranostics | 198 |
| Gold nanotripods | PEG-integrins | <20 | 700 | 670 | 2.02 × 10−16 m2 | — | 3× | 0.3–12.5 nM | M | Photothermal | Tumor contrast | 197 |
• The PA performances of nanostructures are often summarized in the signal enhancement by the contrast agent compared to pre-injection signals. This approach allows comparing the brightness of the contrast agent directly to the surroundings, removing the influence of experimental variables, such as the type of tissue or the specific instrumentation employed.
• The size of the nanostructured contrast agent generally plays a key role in determining their optical properties but also whether accumulation via EPR is possible or not.
• Even though the toxicity and the long-term effect of systemic injection of nanostructures in living animals are rarely investigated in preclinical studies, in vitro biocompatibility and non-cytotoxicity studies are most often conducted prior to in vivo experiments. Minimum or no cytotoxicity must be observed for a nanostructure to be considered as a candidate for PA imaging at the clinical and preclinical levels.
• Usually, the maximum absorption wavelength of the nanoconstruct and the excitation wavelength employed in PA imaging should be as close as possible to maximize the absorption of photons and thus the PA signal. As has already been mentioned, both wavelengths should fall in the biological windows of the NIR region (650–950 nm and 1000–1300 nm) to reduce endogenous contrast and to maximize penetration depth.
• Finally, the injection dose is clearly useful to compare the strengths of PA contrast in vivo, together with the time required for contrast accumulation. It should be evaluated considering toxicity, PA performances in vitro and whether the accumulation is expected via active or passive targeting.
Moreover, some novel multifunctional contrast agents allow for imaging in multiple modalities; this can be done either by covalent binding different components, each with its own contrast ability, or by employing compounds which are intrinsically responsive to multiple imaging techniques, allowing for unambiguous contrast detection. Finally, some of the most promising nanostructured contrast agents display activatable PA activity: contrast can only be observable when some chemical modification of the nanocomposite structure triggers the PA emission by the sonophore.
In Tables 1–3, the most important parameters are reported for a variety of nanostructured contrast agents implemented in in vivo PA imaging.
| Targeting/coating | Size a (nm) | Absorption (nm) | PA excitation (nm) | ε or σb | η | PA enh.d | Injected concentration | A/M e | Toxicity f | Application | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Determined by TEM or DLS. It may refer to the measured hydrodynamic diameter. b Molar extinction coefficient (ε) or absorption cross-section (σ) measured for the nanosystem. c Photoacoustic efficiency. d Enhancement of the PA signal due to the contrast agent compared to the background or control sample. e Activatable (A) or multimodal (M) probes. f Collects the evaluations on particle toxicity, if evaluated by the authors. | ||||||||||||
| Pt–Cu alloy NPs | PVP | 100 | Broad | 808 | — | — | — | 1.34 mg kg−1 | M | 300 μM | Tumor contrast | 207 |
| Bi nanoparticles | PEG-Gd3+ | 46 | 700–900 | 680–900 | — | — | — | 10 mg mL−1 | M | Non-significant | Tumor theranostics | 134 |
| Cu nanoparticles | Neodecanoate | 80 | 603 and 746 | 720–870 | — | — | 3× | 16.99 mg mL−1 | — | Previously reported | Sentinel lymph node imaging | 206 |
| Co9Se8 nanoplates | PAA | 100 | broad | 808 | — | — | — | — | M | 120 μg mL−1 | Tumor contrast | 204 |
| MnMoOx nanorods | C18-PEG | 10 × 40 | 830 | 830 | — | — | 2× | 5 mg kg−1 | M + A | — | Tumor theranostics | 205 |
| Pd@Au nanoplates | PEG | 30 | 700 | 700 | 28.6% | 2.5× | 1 mg mL−1 | M | — | Tumor contrast | 209 | |
| Pd nanosheets | PVP | 16 | 790 | 790 | — | — | 3.4× | 0.8 mg mL−1 | — | — | Tumor contrast | 129 |
| Silver nanoplates | PEG | 25–220 | 550–1080 | 740–940 | — | — | — | 1012 nanoplates in 200 μL | — | None up to 1 mg mL−1 | Tumor contrast | 210 |
| Silver bumpy nanoprobes | SiO2-BSA | 200 | 680 | 680–950 | — | — | 70% | 20 μM | M | — | Sentinel lymph node imaging | 211 |
| TiS2 nanosheets | PEG | 100 | 800 | 808 | 26.8 L g−1 cm−1 | — | 4× | 2 mg mL−1 | — | — | Tumor contrast | 131 |
| Titanium nitride NPs | PEG | 20 | 600–1000 | 808 | 28.6 L g−1 cm−1 | 48% | 1.3× | 2 mg mL−1 | M | — | Tumor contrast | 212 |
| WS2 nanosheets | PEG | 50–100 | 700–1000 | 700 | 23.8 L g−1 cm−1 | — | 6× | 5 mg mL−1 | M | — | Tumor theranostics | 132 |
| Upconversion NPs | α-Cyclodextrins | 29 | — | 980 | — | — | — | 0.5 mg mL−1 | — | None up to 0.2 mg mL−1 | Tissue contrast | 199 |
| Bi2Se3 nanoplates | PVP | 70 | broad | 1064 | — | — | 19.6× | 15.26 mg mL−1 | — | None up to 1 mg mL−1 | Tissue contrast | 208 |
| Black phosphorus QDs | Ti(OTs)4 | 2 | 680 | 680 | 22.2 L g−1 cm−1 | — | 6× | 200 μg in 100 μL | — | Non-significant | Tumor contrast | 201 |
| PEG | 22 | 808 | 680 | 2.1 L g−1 cm−1 | — | — | 2 mg mL−1 | — | Photothermal | Tumor theranostics | 202 | |
| Uranium | PLGA | 335 | 848 | 910 | — | — | 1.8–7.4× | 0.38 nM (2.75 mg mL−1) | — | — | Tissue contrast | 133 |
| CuS nanoparticles | PEG | 11 | 990 | 1064 | 2.6 × 107 cm−1 M−1 | — | — | 6–96 μg mL−1 | None up to 96 μg mL−1 | Deep tissue imaging | 128 | |
| Citrate | 20 | 680 and 930 | 680 and 930 | — | — | — | 0.8 mg mL−1 | A | Non-significant | Tumor contrast | 123 | |
| Cu2−xSe nanocrystals | DSPE-PEG | 7.6 | 1150 | 900 | 2 × 10−18 m2 | — | 4.8× | 800 nM | — | IC50 = 22.5 pmol mL−1 | Sentinel lymph node imaging | 125 |
| CuInS/ZnS | DSPE-PEG | 25 and 80 | 650–750 | — | — | 11.6% | — | 2 mg mL−1 | M | Non-significant | Tumor theranostics | 203 |
| Cu2−xS nanodots | DSPE-PEG | 12 | 1064 | 1064 | — | — | 410× | 10 mg Cu per kg | — | None up to 100 ppm | Tumor theranostics | 122 |
| Targeting/coating | Size a (nm) | Absorption (nm) | PA excitation (nm) | ε or σb | η | PA enh. d | Injected concentration | A/M e | Toxicityf | Application | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Determined by TEM or DLS. It may refer to the measured hydrodynamic diameter. b Molar extinction coefficient (ε) or absorption cross-section (σ) measured for the nanosystem. c Photoacoustic efficiency. d Enhancement of the PA signal due to the contrast agent compared to background or control sample. e Activatable (A) or Multimodal (M) probes. f Collects the evaluations on particle toxicity, if evaluated by the authors. | ||||||||||||
| Carbon nanotubes | Pluronic | 2 | Broad | 740–820 | 5 × 103 M−1 cm−1 (ref. 238) | — | 5× | 0.5 mg mL−1 | M | — | Sentinel lymph node imaging | 140 |
| PEG | 80–100 | Broad | 808 | — | 5× | 2 mg mL−1 | — | Non-significant | Tumor contrast | 217 | ||
| PEG-RGD | 50–300 | Broad | 710–780 | — | 80% | 50 nM | — | Non-significant | Tissue contrast | 216 | ||
| Graphene | BSA | 70 | Broad | 808 nm | — | — | 55% | 1 mg mL−1 | — | Non-significant | Tumor contrast | 146 |
| Fullerenes on SiO2 | PHF | 50–100 | Broad | 785 nm | — | — | 100% | 0.45 mg mL−1 | — | Non-significant | Tumor contrast | 218 |
| Carbon nanoparticles | Citric acid-antibody | 12 | 425 | 680–760 | — | — | — | 7 mg mL−1 | — | Photothermal | Tumor theranostics | 220 |
| — | 70 | 820 | 820 | — | — | 9.2× | 90.3 μg mL−1, 0.238 nM | — | Non-significant | Tissue contrast | 221 | |
| PEG | 7 | 510 | 510 | — | — | 60% | 25% vol. 100 μL | — | Non-significant | Sentinel lymph node imaging | 219 | |
| Polypyrrole NPs | PEG-Gd3+ | 70 | Broad | — | — | — | 3× | 5 mg mL−1 | M | Photothermal | Tumor theranostics | 163 |
| PVA | 46 | Broad | 808 | 2.4 × 1010 cm−1 M−1 | — | — | 2 mg mL−1 | — | Non-significant | Vascular imaging | 161 | |
| Polythiophenes | 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) | 40 | 660–700 | 700–820 | 5.0 × 107 cm−1 M−1 | — | 13.3× | 50 μg | M | Non-significant | ROS imaging | 222 |
| 120–160 | 700–825 | 600–1000 | 250 cm−1 L g−1 | — | −1 | 1.6 μg in 10 μL | — | Non-significant | Vascular imaging | 162 | ||
| DSPE-PEG-folate | 50 | 700–850 | 700–900 | — | — | 4× | 0.3 mg mL−1 | — | — | Tumor contrast | 160 | |
| PEG-phospholipid-Gd3+ | 110 | 670 | 680 | — | — | 3.5× | 0.1 mg mL−1 | M | Photothermal | Tumor theranostics | 157 | |
| PEG-b-PPG-b-PEG | 55 | 840 | 750 | — | — | 2.6× | 0.1 mg mL−1 | — | Photothermal | Tumor theranostics | 158 | |
| DSPE-PEG | 50 | 710 | 800 | — | — | 5.5× | 0.5 mg mL−1 | — | Non-significant | Sentinel lymph node imaging | 166 | |
| Diketopyrrolopyrrole-based | PEG-b-PPG-b-PEG | 11–54 | 744–253 | 750–1064 | — | — | 1.5× | 6 mg mL−1 | — | None up to 0.5 mg mL−1 | Tissue contrast | 226 |
| DSPE-PEG | 68 | 809 | 1000–1200 | — | — | 3.4× | 50 μg mL−1 | M | None up to 150 μg mL−1 | Tumor contrast | 171 | |
| PEG-b-PPG-b-PEG | 40 | 616–665 | 680 | — | — | 4.7× | 30 μg in 120 μL | — | Non-significant | Tumor contrast | 164 | |
| SiO2-PEG | 18 | 680–860 | 680 | — | — | 1.5× | 0.1 mg mL−1 | — | — | Protein sulfenic acid imaging | 225 | |
| DSPE-PEG | 45 | 748 | 710–850 | 76 mL (mg cm)−1 | — | 5.3× | 30 μg in 120 μL | — | Non-significant | Tumor contrast | 213 | |
In vivo applications
In this section, the recent applications of the previously described nanostructured contrast media are highlighted. Herein, we took into consideration only the most significant results of in vivo experiments. Firstly, the most remarkable preclinical results obtained with molecular contrast agents are rapidly summarized, and then each class of nanostructured contrast agents is thoroughly investigated.Small molecule dyes
In 2010, Kim et al. reported how ICG could help in the identification of sentinel lymph nodes (SLNs) and their surrounding vessels for breast cancer early diagnosis.179 To prove this, 5 rats underwent PA imaging in vivo after the injection of 1 mM ICG solution. SLNs were clearly visible in the PA images obtained, with a 6-fold signal enhancement recorded compared to the system before injection. In addition, ICG fluorescence was exploited for dual-modality imaging, leading to the formulation of fluorescence images that were matching the PA results. Later, in 2018, Wilson et al. employed antibody-conjugated ICG for intraoperative photoacoustic and dual fluorescence imaging of breast cancer to help the surgeon during the removal of tumoral tissues for minimizing positive resection margins.180 In 2015, Garcia-Uribe et al. employed methylene blue for the same application in a clinical trial on humans.181 Study participants have been subcutaneously injected with MB in the same breast area as their primary breast cancer. Patients underwent noninvasive PA imaging at the injection site. To correctly differentiate the contrast from the surrounding vessels, PA images have been acquired at two different wavelengths (650 and 1064 nm) and compared, obtaining signal enhancement factors of 1.75 on SLNs in the tumor region. As has already been said, SMDs suffer from some important drawbacks, which drastically limit the employability of molecular contrast agents in their free form. These are (1) fast clearance from the circulatory system due to the immune response of the organism, (2) concentration-dependent optical behavior, caused by intrinsic lipophilicity of polyconjugated chromophores, (3) small optical absorption wavelength and low extinction coefficients. Because of this, the attention of scientific research has been directed towards the employment of specifically engineered nanostructured contrast agents, which can overcome the intrinsic limitations of SMDs.It is worth reporting that the possibility of exploiting different phenomena for the generation of photoacoustic signals upon laser exposure has been lately explored. In particular, in 2012, Wilson et al. proposed that laser-induced vaporization of perfluorocarbon nanodroplets could induce PA contrast in biological systems.182 Even though this could provide efficient PA contrast in vivo, the system requires the employment of small molecule dyes as photoabsorbers, with all the limitations associated with dye-based PA contrast.
Metal and semiconducting nanoparticles
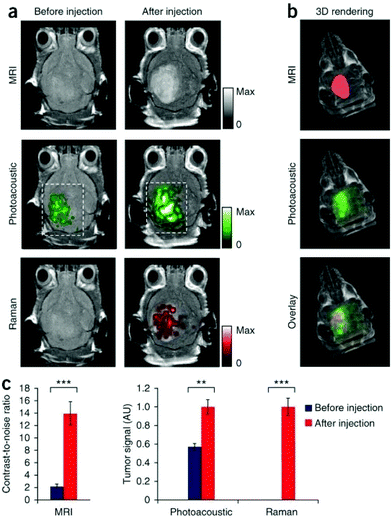 | ||
| Fig. 9 In vivo triple modality imaging of MPR nanoparticles. (a) MRI, PA and Raman 2D axial images before and after injection of MPRs, (b) 3D rendering of MRI and PA signals, and their overlay, showing good tumor contrast, (c) quantification of MRI, PA and Raman signal intensity before and after the injection of the nanoconstruct. Notwithstanding the limitations in PA imaging penetration depth due to the short excitation wavelength employed, thanks to glioblastoma superficiality strong PA contrast has been obtained; adapted from ref. 185. | ||
Lately, Cheng et al. reported the fabrication of a light-triggered assembly of gold nanoparticles which could serve as a contrast agent for PA imaging of tumors.186 The nanoparticles described were composed of a 20 nm gold core functionalized with diazirine moieties on covalently-linked PEG chains. Upon irradiation with 405 nm laser light, diazirine groups cross-link, leading to covalent aggregation. The surface plasmon bands of gold cores shift to the NIR, leading to aggregates with photoacoustic efficiency as high as 78.8%, with negligible cytotoxicity to 4T1 cells up to 200 μg mL−1. After the assessment of the feasibility of laser-induced aggregation in vivo, the authors injected 100 μL of 2 mg mL−1 of AuNPs in the tail vein of female nude mice bearing 4T1 tumors. Tumors have been exposed to 405 nm laser light for 25 s to induce aggregation at the tumor site, followed by PA imaging. Compared to control animals, images obtained from tumor-bearing mice showed increased PA contrast at the tumor site.
Recently, gold nanorods have found various applications on the basis of their photoacoustic response to exploit them for tumor detection in vivo. In 2012, Jokerst et al. exploited localized surface plasmon for SERS (surface-enhanced Raman scattering) on the GNR surface: dual-modality SERS and PA gold nanorods have been used to image subcutaneous ovarian tumor xenograft models (OvCA) in vivo, showing how GNRs could offer both photoacoustic signals for diagnostics and tumor visualization and optical SERS for image-guided resection.187 For this purpose, GNRs have been prepared with the absorption band in the NIR for PA signals and then functionalized with an IR laser dye and thiol-PEG to ensure an intense SERS response. By means of their photoacoustic response, intravenously injected GNRs have been shown to accumulate by the EPR effect in 2008, HEY and SKOV3 OvCA tumor cells, allowing for efficient tumor visualization. On the other hand, SERS has been exploited for tumor edge identification and for image-guided resection on tumor xenografts from the 2008 cell line OvCA model.
Guo et al. recently reported the preparation of gadolinium oxysulphide-coated gold nanorods as dual-modality contrast agents for PAI and MRI, employing the strong MRI contrast of gadolinium, which is strongly paramagnetic due to its seven unpaired electrons.188 GNRs have been prepared with the previously described seeded-growth method, and coating with gadolinium oxysulphide has been performed via a one-step process with Gd nitrate as a gadolinium source in the presence of ascorbic acid (AA) and thioacetamide (TAA) at 80 °C. Then, NPs have been further modified with polyvinyl alcohol (PVA) for stabilization. Increasing the Gd2O2S shell thickness could increase the effective dielectric constant surrounding the plasmonic core, resulting in a red-shift of the longitudinal LSPR mode. The PA and MRI response of the nanosystem has been tested both in vitro and in vivo. Once the feasibility of the study was proved, GNRs@Gd2O2S has been intratumorally injected into Hep G2 tumor-bearing BALB/c nude mice as a model for in vivo PAI/MRI dual-modal imaging. A 4-fold increase in PA signal and a 2-fold increase in MRI contrast have been observed after injection, demonstrating the effect of the system as a dual-modality contrast agent.
An additional interesting application of GNRs in photoacoustic imaging has been described by Du et al.189 The authors demonstrated the possibility of building a nanoplatform by assembling GNRs on the surface of a DNA-origami nanostructure. The nanostructured hybrid, which combines the well-known optical properties of GNRs with the enhanced passive tumor targeting and long-lasting effects of DNA nanostructures, has been exploited as a unique probe and an efficient contrast agent in PAI, generating intense signals at very low concentrations. PAI has been performed on breast-tumor xenografted mice in which DNA-GNRs nanoplatforms have been intravenously injected, revealing enhanced tumor accumulation compared to bare GNRs. Accumulation of gold via EPR effect has been observed to be slower but more persistent with DNA-GNRs compared to bare GNRs, giving rise to elevated tumor/muscle contrast ratio even 24 h post-injection (Fig. 10).
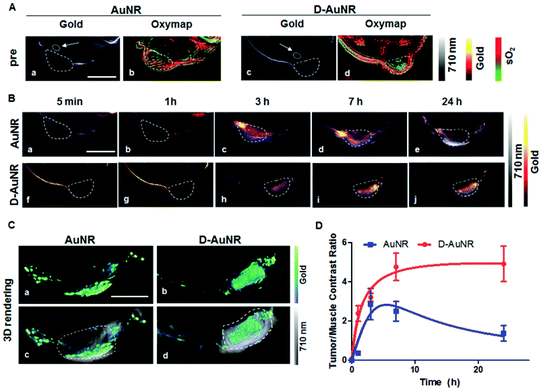 | ||
| Fig. 10 (a) Gold distribution and the corresponding oxygen saturation maps before intravenous injections of GNRs and GNRs-DNA in 4T1-tumor-bearing mice. (b) GNRs and DNA-GNRs distribution after intravenous injection, (c) 3D rendering of the tumor region 24 h post-injection and (d) PA contrast ratio between tumor and muscle for GNRs and DNA-GNRs as a function of time after the injection. Adapted from ref. 189. | ||
In 2018, Chen and co-workers developed polyethyleneimine-modified GNRs (GNRs-PEI) connected to a Fuel Improved microRNA Explorer (FIRE) capable of distinguishing tumor cells from healthy tissues efficiently.190 Their nanoconstruct was able of carry out active targeting, thanks to the detection of microRNA-21 strands, upregulated in cancer cells, by designing a smart fluorescence quenching approach in which the fluorescence signal was expected to be much stronger for tumor tissues than for the healthy one. Their platform demonstrated good tumor inhibition efficacy in vivo via both fluorescence imaging and photoacoustic imaging-guided photothermal therapy. To evaluate PA contrast, 100 μL of 0.8 mg mL−1 GNRs-PEI solution have been intratumorally injected into tumor-bearing mice, revealing strong PA signals that can be easily differentiated from the background at the tumor site.
Recently, we described the fabrication of a core–shell composite nanomaterial in which a paramagnetic Fe3O4 core has been covered with a layer of silica on which a gold shell has been grown.191 The so-called Fe3O4@SiO2@Au NPs have been entrapped in polymeric micelles to protect the metallic core from the biological environment; then they have been further conjugated to folic acid to ensure specific targeting to IGROV ovarian cancer cells. The nanoconstruct, which displayed a hydrodynamic radius of about 165 nm, had its optical absorption maximum between 850 and 900 nm with a high molar absorption coefficient and no fluorescence properties; this made the core–shell system a good candidate for the multispectral PA imaging of tumor models in vivo. Xenograft tumor-bearing mice were systemically injected with folic acid-conjugated Fe3O4@SiO2@Au NPs at concentrations of 8 mg Au per kg, and the tumor was imaged at 860 nm 4 h post-injection for maximum sensitivity. The magnetic core allowed for MRI, making the core–shell system a sensitive dual-modality imaging platform for the detection of ovarian cancer.
As previously introduced, gold nanocages can represent powerful theranostic platforms, and they can give rise to high PA signals, thanks to their surface plasmon features. In 2014, Jeon et al. described the use of NIR-absorbing gold nanocages to image the murine bladder in vivo.192 PEGylated gold nanocages (λ = 700 nm) have been prepared by galvanic replacement reaction on silver nanocubes and covalently linked to PEG-SH for biocompatibilization. After transurethral injection of a 2 nM solution of nanocages, rats underwent non-invasive PA cystography and displayed a 22.4× enhancement of PA signal compared to the control sample. Moreover, no more signal could be observed in the bladder after 24 h, suggesting that the injected nanosystem was not retained in the bladder but washed away by the urine.
Sakthi Kumar and co-workers recently described the production of gold nanocages for the first contrast-based PA imaging of the eye.193 The nanosystem was produced by an ultrafast microwave-assisted procedure, which led to nanocages with a uniform edge size of about 50 nm, showing strong optical absorption properties at 900 nm. After the evaluation of the PA contrast potential in polyethylene tubes for the obtained nanostructure, nanocages have been injected right above the iris of an enucleated porcine eye, showing a PA contrast enhancement of 50% and thus leaving the open possibility of surface modification of nanocages to enable active targeting for specific eye diseases (Fig. 11).
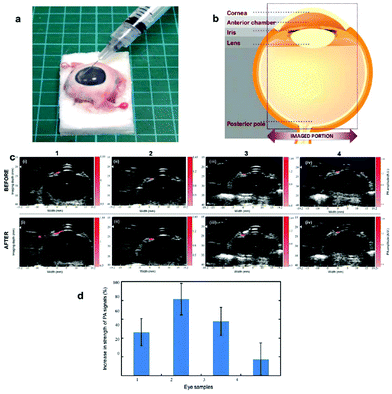 | ||
| Fig. 11 (a) Photograph of the enucleated porcine eye. (b) Schematic representation of the eye parts. (c) PA images acquired before and after the injection of nanocages in four different eye samples, showing increased PA contrast. (d) PA contrast enhancement for the four injected samples. Adapted from ref. 193. | ||
Kim et al. have reported the applications of gold nanocages for molecular PA imaging of melanomas.194 Nanocubes, prepared by the traditional galvanic replacement method, have been conjugated with [Nle4,D-Phe7]-α-melanocyte-stimulating hormone in order to bind to α-MSH receptors overexpressed in melanomas. The authors injected 100 μL of 10 nM targeted Au nanocages into the tail vein of mice; 6 h later, the mice underwent PA imaging at the tumor site with a 778 nm pulsed laser. They observed 300% PA signal enhancement at the tumor site compared to the signal in mice injected with the same amount of untargeted nanocubes, showing both the efficacy of their active targeting system and the efficiency of gold nanocubes for PA contrast. In addition, PA has been employed to image the vasculature surrounding the tumor, obtaining more information about the melanoma state. This has been performed by tissue laser excitation at 570 nm.
In 2016, Bao et al. have shown, in their work, how peptide-conjugated gold nanoprisms (AuNPrs) could serve as a PA contrast agent for tumor angiography and in situ imaging of a murine orthotopic gastric carcinoma model.195 By scanning the tumor site with a PA setup at 710 nm, the tumor vasculature has been visualized before and 1, 3 and 6 h after tail-vein injection of peptide-coated PEGylated AuNPrs at 1 mg mL−1 concentration, showing retention of good contrast at the tumor site. In parallel, antibody-conjugated AuNPrs have been used to image gastric cancer by photoacoustic detection of the accumulation of the gold nanostructure. Since most PA photoabsorbers have potential application in photothermal therapy (PTT), AuNPrs have been exploited to directly measure by PA imaging the reduction in tumor size after prolonged exposure to continuous 980 nm laser light. Their results clearly showed triangular gold nanoprisms as two molecular probes with high sensitivity contrasts and therapeutic agents for tumor detection in vivo, which represent a safe theranostic nanoplatform as confirmed by the histological analysis performed on organ tissues after 14 days from the injection.
The same year, Han et al. described the fabrication of glucose-functionalized gold nanoprisms with improved cellular uptake and cytotoxicity in cancer cells.196 In comparison with target-less nanoparticles, glucose conjugation helped towards the accumulation of photoabsorbing AuNPrs in cancer cells. The authors injected 100 μg of 1 mg mL−1 of glucose-conjugated PEGylated nanoprisms into MGC-803 cell tumor-bearing nude mice. PA imaging of the tumor showed great contrast enhancement, increasing with time post-injection by excitation at 778 nm. A weaker tumor profile was observed after the injection of untargeted PEGylayed nanoprisms, compared to the control image obtained after the injection of PBS solution. The authors explored whether their nanoconstruct could serve as a photothermal therapy agent, showing remarkable results and drastic tumor growth inhibition.
Cheng et al. firstly reported in 2014 the production of 20 nm gold nanotripods as a contrast agent for in vivo photoacoustic imaging.197 Gold nanotripods displayed cross-sections of the order of 10−18 m−2 at 700 nm, generating 33% more contrast than the same mass of gold nanorods. As a proof-of-concept, the authors conjugated cyclic Arg-Gly-Asp-D-Phe-Cys (RGDfC) peptide to PEGylated nanotripods to demonstrate the effective contrast in vivo by active targeting. PA imaging has been performed at 700 nm on living mice after the injection of 100–200 μL of targeted gold nanotripods in PBS, 0.5 to 4 h post-injection. Compared to control experiments, the nanostructure could provide more than 3 times higher PA contrast. Moreover, the signal intensity has been observed to respond linearly to variations of the injected concentration (Fig. 12). Intravenous injection of nanotripods displayed increased contrast at subnanomolar, generating images that well correlated with the corresponding PET studies.
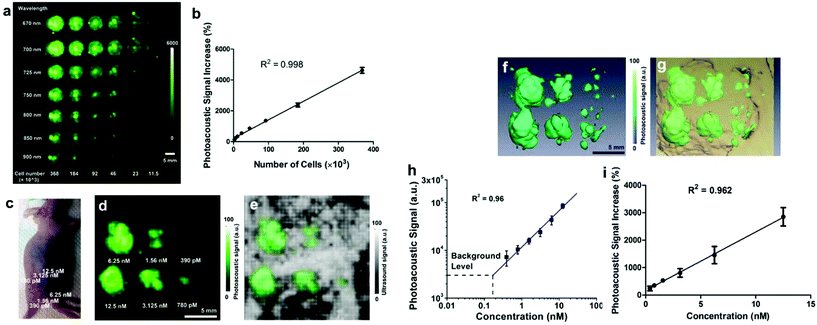 | ||
| Fig. 12 (a) PA images of agar phantoms containing U87MG cancer cells exposed to RGD-conjugated gold nanotripods at different cell concentrations and for different excitation wavelengths. (b) Linear correlation between photoacoustic signal and the number of treated cells in the phantom. (c) Optical picture of a treated mouse, with injection sites highlighted by the injected concentration. (d, e) PA imaging of the area subcutaneously injected with the nanoconstruct, showing the different injection sites with different intensities. (f, g) 3D volume rendering of PA (green) and US (brown) signals recorded. (h, i) Linear correlation between the injected concentration and photoacoustic signal in vivo. Adapted from ref. 197. | ||
Liang et al. reported in 2015 the preparation of highly branched gold nanoparticles named gold nanostars (GNSs) displaying strong PA contrast.198 The nanostructure displayed an absorption maximum at about 635 nm and the hydrodynamic radius has been measured to be around 100 nm. Due to their promising properties, anti-CD44 and anti-CD44v6 monoclonal antibodies have been conjugated to PEGylated nanostars, to evaluate the interaction between the nanoprobes and gastric cancer stem cells (GCSCs). To evaluate PA contrast in vivo, the authors intravenously injected 150 μL of gold 0.867 mg mL−1 as conjugated gold nanostars, and monitored the PA signal intensity at the tumor site over time. Results showed a contrast enhancement by a factor 4.7 and 2.5 for nanostars targeted with CD44v6 and CD44, while only a factor of 1.75 was observed after the injection of untargeted GNSs, showing the reliability of both the contrast from nanostars and the effectiveness of the active targeting.
In summary, gold nanostructures can be considered as some of the most promising nanostructured contrast agents for PA imaging, offering wide shape- and size-dependent absorption behaviour, high photoacoustic conversion efficiencies and well known biocompatibility. They also show high extinction coefficients thanks to the localized surface plasmon resonance (LSPR) phenomena, which allows the employment of low injected concentrations to achieve efficient contrast in vivo. Moreover, since their surface chemistry is well known and deeply explored in the literature, gold nanostructures can be easily functionalized either with active targeting agents or with additional functional probes, which allows their employment for tumor imaging in multiple modalities, such as MRI, CT, PET, SERS and optical imaging. Amongst other gold nanostructures, the most promising results have been obtained with gold nanorods (GNRs): their longitudinal LSPR can be finely tuned in the synthesis step throughout the NIR, allowing us to specifically design GNRs to overcome the different endogenous contrast in different biological tissues.
Quantum dots and rare-earths
Upconversion nanoparticles (UCNPs) are Yb3+ and Er3+ co-doped NaYF4, which are characterized by efficient NIR-to-visible optical conversion, thanks to energy transfer processes between Yb and Er. In 2014, Maji et al. described how luminescence from UCNPs could be quenched to ensure high PA contrast in vivo upon irradiation at 980 nm.199 Lipophilic UCNPs displayed the characteristic emission bands from Er3+. By encapsulating the doped nanoparticles in α-cyclodextrins, hydrophilic UCNPs (UC-α-CD) have been obtained. Hence, the quenching effect of water as a solvent strongly reduced the luminescence of the particles in favour of a remarkable PA contrast enhancement. PA images have been acquired before and 35 min after the intravenous injection of 250 μL of UC-α-CD at 500 μg mL−1 concentration. Results showed signal retention enhancement at the kidney while the cytotoxicity tests revealed the non-toxicity of the nanostructure up to 200 μg mL−1, supporting the applicability of rare-earth doped semiconducting nanoparticles in photoacoustic imaging by effective water quenching.The same year, Sheng et al. reported the synthesis of rare-earth doped NaYF4 with different morphologies for similar applications.200 These authors intravenously injected NaYF4:Er,Yb with different surface stabilizers in living mice, declaring a signal enhancement of 2.7 times the background by excitation at 975 nm.
Lately, Sun et al. described how TiL4-coordinated black phosphorus (TiL4@BP) quantum dots could serve as a contrast agent for in vivo PAI of cancer, as an improvement of a previous study from 2016.201,202 The QDs’ average size was 2.8 nm, and they displayed molar extinction coefficients of 22.2 L g−1 cm−1 at 680 nm, 3.6 times higher than the one measured for GNRs when compared on a per mass basis. To evaluate the effective PA contrast that could be generated in vivo, 200 μg of TiL4@BP QDs have been intravenously injected into eight MCF-7 tumor-bearing Balb/C nude male mice. PA images have been obtained by summing the PA response acquired from five laser pulses at 608 nm in 15 nm steps before and 0.5 to 48 h post-injection. The obtained images demonstrated the effective accumulation of TiL4@BP QDs in the tumor, with a maximum contrast obtained 4 h post-injection and still weakly retained after 48 h from the administration. No relevant cytotoxicity has been detected in vitro.
Lv and coworkers reported in 2016 the NIR-emission properties of CuInS/ZnS (ZCIS) QDs for both tumor fluorescence and photoacoustic imaging.203 The authors prepared two similar nanosystems with different sizes (25 and 80 nm) to evaluate differences in the diffusion profiles and tumor uptake. The QDs displayed strong optical absorption at 650–750 nm, suggesting a potential PA imaging ability. Multispectral Optoacoustic (MSOT) imaging was performed between 680 and 900 nm at different time steps after the intravenous administration of 200 μL of 2 mg mL−1 ZCIS solution in nude mice bearing 4T1 tumors. The experiments revealed size-dependent particle accumulation and cleavage at the tumor site: smaller particles have longer tumor retention times and tumor uptake compared to the bigger ones (Fig. 13). In addition, fluorescence from the QDs allowed for fluorescence imaging, which confirmed the results from PA imaging. Furthermore, the ability of ZCIS to mediate photoinduced tumor ablation was evaluated with success.
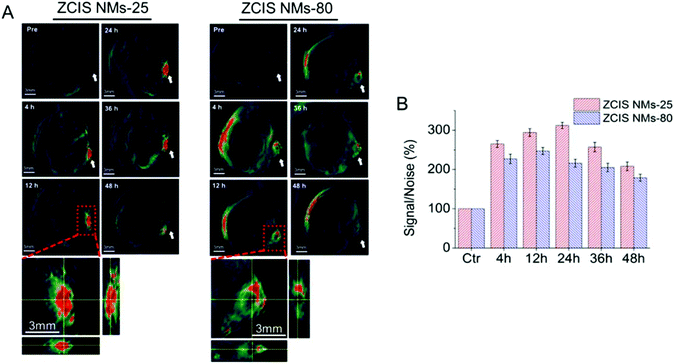 | ||
| Fig. 13 (A) In vivo MSOT imaging of 4T1 tumor-bearing nude mice injected with ZCIS of 25 nm (NMs-25) or 80 nm (NMs-80) at different time steps after the injection, revealing higher contrast at the tumor site for smaller particles. (B) Average MSOT signal intensity at the different time steps. Adapted from ref. 202. | ||
To sum up, nanosystems containing or composed of this exotic class of materials display good photoacoustic contrast and most of them can be also employed for fluorescence imaging due to their widely reported photoemission behaviour. There are still few examples of their application in PA imaging; great effort still needs to be directed towards the study of their bioaccumulation and toxicity; notwithstanding their excellent and tunable photophysical properties, quantum dots and rare-earth nanoparticles still suffer from poor biocompatibility and high production costs which, nowadays, drastically limit the potential translation of this class of nanosystems into the clinics.
Lately, Gong et al. described the production of PEGylated MnMoOx nanorods as a PA probe for the identification of glutathione (GSH) in vivo.205 GSH is an easily oxidizable tripeptide with good antioxidant properties. Their nanoprobe was capable of actively targeting without the need for any surface modification but exploiting the chemistry of the core elements themselves. In fact, GSH was able to reduce MoVI to MoV efficiently, thus causing the disruption of the non-absorbing nanorod and providing contrast from strongly absorbing nanodots. Since GSH is overexpressed in various tumor tissues, their system was able to provide efficient PA contrast in vivo at the tumor site by excitation at 830 nm, revealing contrast capability triggered by the tumor itself. Moreover, the release of Mn2+ ions during GSH activation allowed for the possibility to employ T2-weighed MRI to co-detect tumor localization. To further study the potential of the nanosystem for GSH detection, mice bearing 4T1 tumors were intravenously injected with PEGylated MnMoOx, revealing 2× PA signal enhancement at the tumor site compared to healthy muscle tissue. This work reports a smart approach for the generation of tumor-induced PA and MRI contrast, since it is the tumor itself that chemically modifies the small nanorods through the overexpression of GSH. Here, intravenous injection of the nanorod dispersion allows for PA contrast at the tumor site without the need for any active targeting; however, the conjugation of active targeting species such as antibodies, nucleic acids or small targeting molecules could, in principle, increase the specificity of the contrast and reduce the distribution of manganese- and molybdenum-based compounds in the body.
Pan et al. have reported the synthesis of copper neodecanoate nanoparticles for PA imaging of SLNs.206 In their work, the authors for the first time produced a copper-based contrast agent for SLN detection by PA imaging in vivo. Their nanoconstruct was an 80 nm wide self-assembled copper neodecanoate nanoparticle encapsulated in phospholipid micelles, which displayed an optical absorption maximum at λ = 603–746 nm. The absorption is believed to originate from crystal field splitting of Cu2+-neodecanoate complexes, and it has been thought to be exploitable for PA imaging. After the administration of 1 mg mL−1 nanoparticle suspension in living mice, bright PA contrast was observed immediately and 60 min post-injection. A signal enhancement of 500% has been measured for a 20% colloidal suspension of nanoparticles, much faster accumulation rates compared to GNRs. However, compared to most other reviewed nanostructured contrast agents, copper neodecanoate nanoparticles display a quite blue-shifted absorption (below 800 nm) which can severely limit the PA response of the nanosystem in deep tissues.
In 2016, Zhou et al. developed dendritic Pt–Cu alloy nanoparticles (DPCNs) as multimodal imaging and guided chemophotothermal therapy (CTP).207 Furthermore, they demonstrated that their construct could serve as light and a pH-triggered drug delivery platform, and the promising photothermal properties of DPCNs suggested their implementation for PA imaging and CTP. Thanks to their elevated surface area, dendritic structures can adsorb an increased amount of small molecules, improving their performances in drug loading capacity. Firstly, negligible cytotoxicity was observed up to 300 μM; therefore, tumor-bearing mice were intravenously injected with DPCN at a dose of 1.34 mg kg−1 to undergo PA imaging analysis. Imaging results showed efficient nanoparticle accumulation in the tumor and strong contrast enhancement at different time steps after injection (0 to 24 h).
Lately, Park et al. reported the novel synthesis of Bi2Se3 nanoplates for long-wavelength PA imaging of sentinel lymph nodes in vivo.208 Thanks to their absorption after 1000 nm, and thus the employment of low-energy highly penetrating radiations, PA imaging of deep tissues has been explored. After the complete evaluation of the photophysical properties of the exotic material, PA imaging contrast has been first evaluated ex vivo in deep chicken tissues by excitation at 1064 nm (ND:YAG laser); then it has been employed to image small animals. In parallel, 50 μL of 15.26 mg mL−1 nanoplates have been transurethrally administered to Balb/c mice for contrast-based cystography; 200 μL of the same solution has been orally given to mice for the imaging of the gastrointestinal tract and an additional 20 μL have been subcutaneously injected to image sentinel lymph nodes. For all the investigated tissues, strong PA signal enhancement was observed, with high signal-to-noise ratio and reduced cytotoxicity. In this study, the authors reported very strong PA signal enhancement both in the bladder and at the lymph nodes, but it should be noted that the injected concentration is relatively high (over 15 mg mL−1); thus, detailed toxicity and bioaccumulation studies should be performed to evaluate the impact of high concentrations of bismuth and selenium on the health of treated mice.
Chen et al. described in 2014 a novel nanostructured theranostic platform based on core–shell Pd@Au nanoplates for in vivo PA imaging, CT imaging and PTT.209 Hexagonal palladium nanosheets with an average diameter of 26 nm were covered with a tunable shell of gold for optical absorption properties in the 600–1300 nm region. To quantify PA contrast from Pd@Au nanoplates, Balb/c mice were injected with 300 μL of 1 mg mL−1 PEGylated nanoplate solution and PA images have been recorded at 1 to 24 h post-injection. After prolonged accumulation by the EPR effect, nanoplates generated contrast 4 times brighter at the tumor site than the tissue before the injection (Fig. 14).
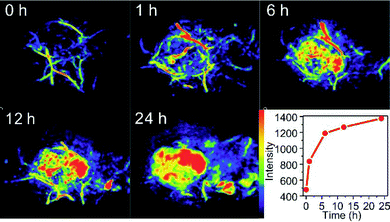 | ||
| Fig. 14 PA imaging of tumors in mice before and 1, 6, 12 and 24 h after the intravenous injection of PEGylated Pd@Au nanoplates. Adapted from ref. 209. | ||
In 2012, Homan et al. first reported the application of silver nanoplates as a contrast agent for molecular PA imaging.210 The authors prepared triangular Ag nanoplates by a seeded-growth method with tunable LSPR from the visible up to the NIR. To detect by PA imaging the active-targeted accumulation of nanoplates in tumors, an anti-epidermal growth factor receptor (a-EGFR) antibody was conjugated on PEGylated nanoprisms, successively injected into the tail vein of Nu/Nu transgenic mice with xenograft human pancreatic cancer grown orthotopically. At 4 h post injection, the maximum accumulation in the tumor was observed by monitoring PA signal over time. Overall, this study revealed the applicability of Ag nanoplates as a PA contrast agent and their potential use for bioimaging and sensing.
Lately, Cha et al. have described the preparation of silica-coated silver bumpy nanoshells (AgNSs@SiO2) for SERS and PA imaging of SLNs in vivo.211 AgNSs have displayed strong scattering properties in the NIR with an absorption peak at 680 nm, and higher SERS enhancement compared to gold nanorods or nanospheres. For this reason, AgNSs have been first labeled with various Raman probes and then embedded in SiO2 shells and surface-stabilized with bovine serum albumin (BSA) for PA imaging at 680 nm and SERS. PA has been performed on female rats, enabling contrast at the SLN 3.3 and 3.8 times more intense than the response of hemoglobin from the surrounding vessels at 40 and 160 min of the injection, respectively. SERS results confirmed strong accumulation on SLN in vivo. Nano-silver has been widely studied in the last few decades thanks to its well-known biocompatibility and antibacterial properties; herein, it has been demonstrated how, similarly to gold, its longitudinal LSPR can be finely tuned in the NIR for application in PA imaging and photothermal therapy. However, nanostructures of this metal require larger sizes and more complex shapes to achieve high absorption coefficients in the biological windows.
In 2017, He et al. documented the application of titanium nitride nanoparticles (TiN NPs) as a novel theranostic platform for PA imaging and PTT.212 Compared to gold and silver, the LSPR of titanium is significantly red-shifted and strong absorptions in the NIR can be obtained without the need for complex architectures.213 The authors obtained 20 nm particles with broad absorption in the NIR and poor cytotoxicity in vitro and suggested their application in PA imaging and PTT. For these reasons, tumor-bearing mice were injected with PEGylated TiN NPs in their jugular vein and PA imaging was performed at the tumor site. PA images showed 150% signal enhancement at the tumor site 24 h after the injection, suggesting effective tumor accumulation. PTT results confirmed the potential of Pt as a theranostic platform for PA imaging.
Carbon nanomaterials
Basic work on the application of single-walled carbon nanotubes (SWNTs) for PA imaging was performed by de la Zerda et al. between 2008 and 2012.214–216 Firstly, it has been described how RGD-conjugated carbon nanotubes could serve as a contrast agent for PA imaging of tumors in vivo. They obtained SWNTs of 1–2 nm in diameter and 50–300 nm in length, which showed strong NIR absorption between 690 nm and 790 nm and linear correlation between concentration and PA signal intensity in vitro. Hence, to evaluate the effective PA contrast obtainable in vivo, 200 μL of 1.2 μM RGD-conjugated SWNTs have been injected into the tail vein of U87MG xenograft tumor-bearing mice. PA 3D images acquired before and 4 h after the injection showed an 8-fold increase in the PA signal compared to the system pre-injection. Successively, in 2010, they reported an improvement of their contrast thanks to the conjugation of ICG to nanotubes. This has allowed a strong increase in the PA contrast in vivo (300×) compared to their previous work, but in this case the signal mainly originates from abundant ICG on the SWNT backbone. Finally, in 2012, the authors further extended their studies on the comparison between nanotubes conjugated to different NIR dyes for in vivo PA experiments, which revealed that stronger NIR absorber dyes allowed for brighter PA contrast.Later, Chen et al. presented PEGylated single-walled carbon nanohorns (SWNHs) with improved stability and biocompatibility as a photothermal agent and for PA imaging both in vitro and in vivo.217 Their SWNHs presented broad and intense absorption in the NIR, well-suited for PA imaging and PTT. After the assessment of their non-toxicity, the authors injected 200 μL of 2 mg mL−1 PEGylated carbon nanohorns in tumor-bearing mice and PA signal was monitored at the tumor site before and up to 48 h after the injection. The PA signal reached its maximum 24 h post-injection with a signal enhancement factor of around 6, suggesting the involvement of slow processes, such as EPR, in the accumulation of contrast in the tumor (Fig. 15). In addition, the proposed nanoconstructs showed remarkable results as PTT agents, thanks to their high light-to-heat conversion efficiency, also exploited in PA imaging.
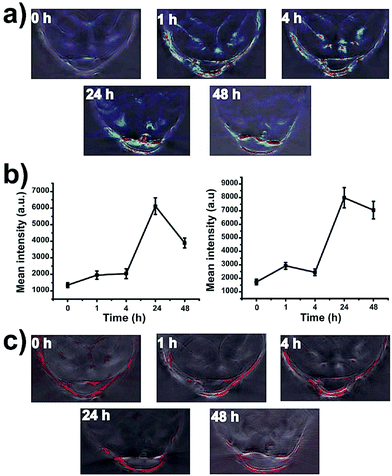 | ||
| Fig. 15 In vivo PA images of tumor bearing mice intravenously injected with PEGylated SWNHs. (a) PA signal from nanohorns before and 1 h, 4 h, 24 h and 48 h after the injection, showing clear accumulation in the tumor. (b) The mean PA signal intensity at the tumor site over time. Maximum accumulation was observed 24 h after the injection. (c) PA images of hemoglobin (Hb) in the tumor at different time steps after the administration. Adapted from ref. 217. | ||
Fullerenes appeared in the field of PA contrast agents when Krishna et al. proposed it in 2010 using polyhydroxyfullerenes (PHFs) for PA imaging of tumors.218 Water-soluble PHF and chitosan-encapsulated PHF nanoparticles have shown good PA contrast in vitro and negligible cytotoxicity, suggesting their potential use in PTT and PA imaging in vivo. Hence, BT474 tumor cell-bearing mice have been intratumorally injected with 30 μL of PHF 10 mg mL−1 or 0.45 mg mL−1 of PHF-functionalized chitosan nanoparticles before undergoing PA imaging analysis in the tumor region. Both injections showed good contrast at the tumor site compared to control experiments, generating signals as high as 4–5 times the endogenous response.
Notwithstanding their promising contrast abilities, good absorption coefficients and low reported toxicity, both carbon nanotubes and PHFs display broad absorption throughout the visible and the NIR: this can hamper the employment of multispectral approaches for the discrimination of exogenous contrast from the endogenous one, generating wavelength-independent contrast.
Graphitic carbon nanoparticles from natural sources were prepared in 2013 by Wu et al. from honey in a domestic microwave.219 The authors microwaved a dispersion of one macromolecular passivating agent (PEG or polysorbate) in honey under an Ar atmosphere for 10–30 min, obtaining 7 nm luminescent carbon nanoparticles or carbon dots (OCNs). The particles showed broad absorption throughout the NIR; thus their PA properties towards the identification of SLNs have been tested. After the assessment of the in vitro potential of carbon dots as PA contrast agents, 650 nm was chosen as the best excitation wavelength to maximize the signal-to-noise ratio. 1–2 min after the intravenous injection of 100 μL of 25%vol OCNs solution into living mice, PA images of the femoral vessels have been acquired in vivo, showing a 60-fold PA signal enhancement at the SLN right after the injection, which rapidly decreased due to poor nanoparticle retention at the lymph node.
Recently, Wu et al. deeply investigated PA contrast generated by porphyrin-containing carbon nanodots (C-Dots).220 In particular, the authors conducted a partial hydrolysis of 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (TAPP) and citric acid to afford carbon dots with strong UV-visible and NIR absorption. Reactive carboxylic moieties from citric acid were observed to be directed towards the surface of the particles, allowing for EDC-assisted amide coupling with α,ω-diamino PEG. Hence, the carboxylic terminus of the C225 antibody has been coupled to the C-Dots surface to exploit the high binding affinity of the antibody for the epidermal growth factor receptor (EGFR), allowing for active targeting. PA imaging analysis in vitro revealed that deprotonation of the porphyrin induced by alkaline pH completely inhibits the light absorption properties of the system, suggesting the possibility of obtaining PA contrast triggered by the intrinsic acidity of cancer cells.
As has been reviewed in detail, graphitic carbon nanostructures offer good PA contrast and improved biocompatibility, despite displaying visible rather than NIR absorption and very small size. Moreover, the molar absorption coefficient and photothermal conversion efficiency of carbon nanoparticles are rarely evaluated, making it hard to compare their performances with different nanostructures showing similar physical–chemical features.
Finally, nanodiamonds have been studied by Zhang et al. for their potential application as a PA contrast agent of tissues.221 Nanodiamonds (NDs) have been prepared by ion irradiation of natural diamond powder at high dosage to create the vacancies required for electronic transition, and thus absorption, to occur. After in vitro and ex vivo PA imaging tests to assess the pure contrast from nanodiamonds, in vivo experiments were conducted. NDs have been subcutaneously injected into the lower back of a mouse, revealing 919% signal enhancement at the injection site and 567% contrast for a 3 mm deep injection. However, despite the promising results about nanodiamonds’ cytotoxicity, the complexity of the synthesis procedure makes NDs hardly employable in the clinics on a large scale.
Polymeric nanostructures
In two consecutive studies from 2014 and 2015, Pu et al. amply described the performances of engineered semiconducting polymer nanoparticles (SPNs) for preclinical PA imaging.222,223 In their first work, they reported a NIR-activatable PA probe for the detection of the progression of pathological processes. Poly(cyclopentadithiophene-alt-benzothiadiazole) had been chosen to build SPNs by surfactant-assisted nanoprecipitation because of its strong absorption in the NIR, suitable for PA and fluorescence imaging. The nanoparticles displayed an absorption peak at 660 nm, with a mass absorption coefficient of 93 mL mg−1 cm−1. The PA performances of SPNs have been evaluated in comparison with GNRs and SWNTs, both in vitro and in vivo; 50 μg of nanoparticles were injected into mice for lymph node tracking by PA and fluorescence detection. The recorded amplitude of the PA signal increased by a factor of 10 after administration, allowing the clear visualization of several lymph nodes in the region of injection, confirmed by fluorescence imaging from the SPNs. The obtained PA amplitudes for SPNs have been found to be 4 times greater than the ones recorded for GNRs and SWNTs on a mass basis (Fig. 16).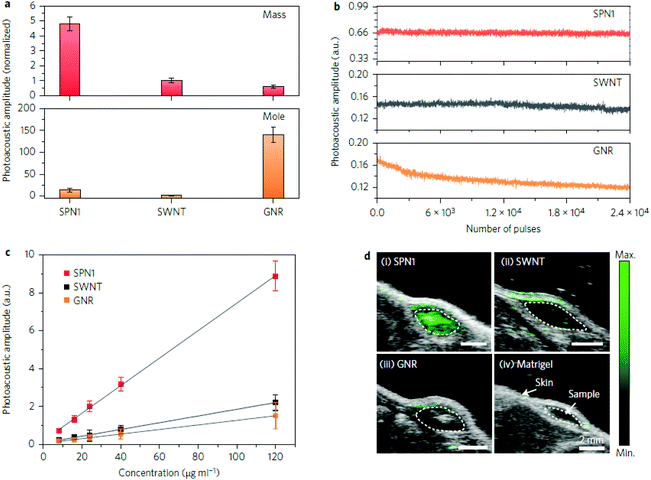 | ||
| Fig. 16 Comparison between the PA performances of SPNs, gold nanorods (GNRs) and SWNTs. (a) Normalized PA amplitude by mass or by mole. (b) Nanoparticle signal stability with prolonged irradiation for the three systems. SPNs display constant PA emission, while GNRs are less stable due to reshaping phenomena. (c) Calibration curves for PA contrast vs. concentration in vitro. (d) Recorded PA images at the site of injection of Matrigel-included nanoparticles at 8 μg mL−1 concentration. Adapted from ref. 222. | ||
Moreover, by nanoprecipitating a semiconducting polymer with IR775S, a NIR dye sensitive to reactive oxygen species (ROS), the authors built a smart PA nanoprobe whose PA spectrum was sensitive to endogenously generated ONOO- and OCl- in vivo. Later, the same authors reported a new series of diketopyrrolopyrrole-based SPNs for in vivo PA imaging of tumor, as a proof-of-concept. The SPNs with the best performances have been chosen out of 3 different polymers for PA imaging in vivo, revealing strong contrast at the tumor site up to 24 h post-injection. Remarkable results were obtained by Huynh et al. in 2015 when they described the production of convertible porphyrin microbubbles (pMBs) for multimodal PA/US imaging.224 They first built micellar microbubbles encapsulating perfluorocarbon gas using the porphyrin-containing bacteriochlorophyll–lipid (BChl-lipid), generating a microparticle displaying US contrast due to the high acoustic impedance of gasses and PA and fluorescence imaging due to absorption from porphyrin groups. Moreover, by exposure to low-frequency US waves, the microbubbles were transformed into smaller particles (5–500 nm) with the same optical properties as the former. After the stability of the PA contrast had been assessed in vitro, tumor-bearing mice were injected with 150 μL of 8.4 × 107 pMBs solution to study and verify PA contrast and the micro-to-nano transformation by PA imaging.
More recently, Lyu reported a new theranostic nanoplatform based on semiconducting polymers for PA imaging and PTT.225 Molecularly-engineered poly[2,6-(4,4-bis(2-ethylhexyl)-4H-cyclopenta-[2,1-b;3,4-b′]dithiophene)-alt-4,7-(2,1,3-benzothiadiazole)] (PCPDTBT) and (6,6)-phenyl-C71-butyric acid methyl ester (PC70BM) nanoparticles have been prepared by nanoprecipitation. PCPDBTB is a semiconducting polymer with absorption in the NIR (840 nm), while PC70BM is a highly electrophilic and hydrophilic fullerene that enables intramolecular PET contrast. Thanks to HOMO–LUMO coupling between the polymer and the fullerene units, increasing amounts of fullerenes caused sensitive increment of PA signal in the NIR. Hence, 200 μL of 100 μg mL−1 nanoparticle solution have been systemically injected into 4T1 tumor-bearing mice, and PA images have been acquired 6 h post-injection. Images revealed 1.8× PA signal enhancement for SPNs compared to the injection of control nanoparticles. The nanoconstruct also allowed for fluorescence imaging and PTT showing promising results as a carbon-based biocompatible theranostic platform.
The same year, Jiang described semiconducting polymer nanoparticles with absorption in both the biological windows in the NIR.226 SPNs were built by nanoprecipitation of a mixture of poly[diketopyrrolopyrrole-alt-thiophene] and poly (diketopyrrolopyrrole-alt-thiadiazoloquinoxaline) with PEG-b-PPG-b-PEG as a stabilizer. The obtained SPNs displayed broad NIR absorption at 744 nm and 1253 nm, allowing for NIR dual-wavelength PA imaging for more accurate localization in living tissues. As a proof-of-concept, in vivo PA imaging of the rat brain vasculature was performed on a homemade PA system equipped with two lasers, at 750 nm and 1064 nm. PA signal remarkably increased at 750 nm after the intravenous injection of 300 μL of 6 mg mL−1, but contrast at 1064 nm was 1.5 times stronger. These results demonstrate that SPNs can serve as a PA contrast agent in the second biological window of the electromagnetic spectrum, where high penetration depth can be achieved.
To sum up, semiconducting conjugated polymers offer the possibility to employ full-organic self-assembling materials for generating efficient PA contrast in vivo in a wide spectral range. However, thanks to the rigid conjugated structure of the polymers, fluorescence phenomena widely occur upon NIR laser excitation. Therefore, detailed studies on the photothermal conversion efficiencies should be conducted on different SPNs to evaluate their effective PA contrast ability. Moreover, detailed studies on the possible metabolic pathways involving SPNs should be conducted to evaluate the possible toxicity of cleaved monomers circulating in the body.
As a first application of protein-based PA contrast, Li et al. developed a genetically encoded small NIR photochromic protein capable of a strong PA effect, and a novel approach for the application of PA imaging to the study of protein interactions in vivo.227 In this work, the authors employed temporal unmixing to discriminate PA signals from cells distributed at different depths in biological systems. The different photophysical behavior of two different long-wavelength absorbing bacterial phytochromes, RpBphP1 from Rhodopseudomonas palustris and DrBphP-PCM from Deinococcus radiodurans, have been exploited to develop a reversibly switchable PA computed tomography system (RS-PACT) combined with single-impulse panoramic PA imaging (SIP-PACT) and real-time detection of genetically encoded NIR-absorbing protein probes. Briefly, U87 cells expressing the bacterial phytochrome DrBphP-PCM have been injected in the right lobe of a living mouse liver, followed by the injection of HEK-293 cells, engineered to express both the bacterial phytochromes in the left lobe of the organ. By exploiting the different decay profiles of the PA signal of the two proteins over time, signals coming from the different photoemitters have been efficiently separated. Furthermore, DrBphP-PCM has been split into two of its main components, named DrPAS and DrGAF-PHY, to achieve PA contrast only when the two domains reconnected to form the photoresponsive core. By connecting the two distinct domains to different interacting proteins and exploiting the photoswitching properties of the engineered system, the split phytochrome DrSplit has been employed to efficiently study protein–protein interactions (PPI) in vivo.
Hybrid nanocomposites
In this last section, we review complex hybrid nanosystems composed of two or more different nanostructured sub-units, capable of efficient PA contrast. These represent the most advanced developments in the field, allowing for extremely tunable responses and enhanced contrast due to the combination of the thermal expansions of the single components.In 2009, Kim et al. reported the preparation of golden carbon nanotubes (GNTs) as a biocompatible theranostic platform for simultaneous PA-PT microscopy to overcome the limitations in toxicity and absorption for carbon nanomaterials by taking advantage of the strong optical absorption and consolidated biocompatibility of gold.228 The authors prepared GNTs by depositing a 4–8 nm thick gold layer onto SWNTS by slow reduction of HAuCl4 with no need for external reductants. Similar to GNRs, GNTs showed longitudinal and transverse LSPR absorption, at 520–530 nm and 850 nm, respectively, comparable with gold nanorods with an aspect ratio of around 4–5. GNTs have been further conjugated with an anti-LYVE-1 antibody for the evaluation of actively targeted accumulation of contrast in vivo. Anti-LYVE-1 had been previously reported to specifically bind lymphatic endothelial cells (LECs) lying on the internal surface of lymphatic vessels; thus conjugation should have ensured contrast from lymphatic vessels. After the introduction of the GNTs into the lymphatic system of living mice, simultaneously acquired PA/PT microscopic images revealed a 70-fold enhancement in the PA signal at its maximum (1 h post-injection), allowing for noiseless visualization of lymphatics in the mesentery.
In addition, Liu et al. have in 2013 described the implementation of Au–Cu2−xSe heterodimer nanoparticles as a plasmonic–semiconducting hybrid contrast agent for in vivo PA imaging.229 Nanoparticles have been obtained using gold nanoparticles as seeds for the seeded growth of Cu2−xSe nanocrystals. The interaction between plasmonic and semiconducting domains generates broad absorption across both the visible and the NIR, up to 1750 nm. The change in PA signal was monitored in the axillary vasculature rats (at 1064 nm) after successive intravenous injection of 200 μL of 4 mg mL−1 Au–Cu2−xSe nanocrystals, showing 200% contrast enhancement 1 h after the first injection and 290% 1 h after the second injection.
Later, Wang et al. employed PA imaging for the detection of gastric cancer using RGD-conjugated SiO2-coated gold nanorods (sGNRs) on the surface of multiwalled carbon nanotubes (MWNTs).230 The two nanostructures have been coupled via amide bond formation between amino groups on sGNRs and the carboxylic residues on MWNTs. The absorption spectrum of MWNTs/sGNRs displayed broad absorption in the NIR, with the LSPR peak from GNRs still present at 680 nm. Contrast efficiency was tested in vivo by PA imaging of a subcutaneous gastric cancer xenograft model in mice, showing contrast enhancement and accumulation at the tumor site up to 12 h post-injection. Moreover, 20% PA signal enhancement has been confirmed to be a consequence of sGNR interaction with MWNTs at the quantum level in the hybrid nanostructure.
The same type of interaction at the nanoscale was also explored later on when Moon et al. described their experiments with reduced graphene oxide coated gold nanorods (r-GO-GNRs) for PA imaging.231 In their work, the authors studied the enhanced NIR absorption properties (λmax ≈ 750 nm) of r-GO-GNRs and confirmed their excellent photothermal stability and photoacoustic efficiency. Furthermore, to prove the promising PA contrast in vivo, 50 μL of 1.25 nM r-GO-GNRs have been dispersed in Matrigel and injected into the back of mice. PA imaging at the injection site (at 750 nm) revealed a 40-fold signal enhancement from the composite nanostructure (Fig. 17).
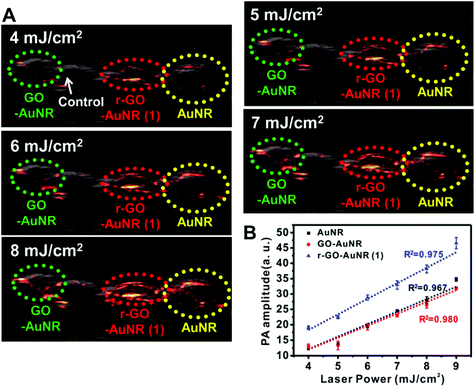 | ||
| Fig. 17 In vivo PA imaging experiments on GNRs, GO-GNRs and r-GO-GNRs. (A) PA images of the injection sites obtained by changing the laser power from 4 to 9 mJ cm−2. (B) Linear relationship between PA amplitude and laser power; r-GO-GNRs display stronger PA amplitude and higher sensitivity towards variations in laser power. Adapted from ref. 231. | ||
Then, Gao in 2016 reported the PA performances of a different Au-graphene hybrid as an activatable theranostic agent.232 Their nanoconstruct was based on the GO-Au nanoparticle nanocomposite that was covalently connected to the matrix metalloprotease-14 (MMP-14) labeled with a NIR dye. PA imaging revealed a significant contrast in vivo compared to AuNPs and GO alone, as reported previously. The PA enhancement due to the coupling between gold and carbon is believed to be due to fluorescence quenching from gold towards GO, which causes an increase in the photoacoustic efficiency of the graphene sheets. More recently, Chen et al. described the preparation of composite nanomodulators for dynamic contrast-enhanced PA imaging.233 The authors prepared poly(n-isopropylacrylamide) (PNIPAM) nanogels loaded with GNRs and CuS QDs. The two nanosystems have been compared on several aspects, such as optical cross-section, absorption tunability, size and photostability. Dynamic contrast-enhanced imaging allows separating static background signals from dynamic signals from the contrast, hence increasing the signal-to-noise ratio and allowing for unambiguous identification of the contrast.
In 2018, we reported the production of a triple-modality imaging probe based on MnFe2O4 nanoparticles, GNRs and fluorescein, allowing for unambiguous contrast detection in vivo.234 Silica-coated magnetic nanoparticles have been functionalized with amino- and azido-modified silanes, further coupled via click chemistry to alkyne-functionalized GNRs, to achieve a smart assembly capable of triple fluorescence, MRI and PA imaging contrast in vivo. We evaluated PA signals by injection of a few microliters of a nanoprobe solution in chicken breast tissue, revealing good contrast and high signal-to-noise ratio even at concentrations as low as 34 μM, which corresponded to a 50-fold PA signal enhancement compared to the background. Moreover, the PA signal coming from the thermoelastic expansion of GNRs was observed to be constant with time, revealing no reshaping involving the gold nanostructure.
The outstanding properties of these latest examples demonstrate the possibility to covalently combine the PA features of different nanostructures to nanomaterials that display very different properties, allowing for the fabrication of advanced nanocomposites with unique diagnostic efficacies. However, the interactions of the combined nanosystems in a biological environment should be evaluated in each case, and the stability of the connections between independent blocks must be ensured to avoid leakage of some of the components of the hybrid nanocomposites.
Conclusions
In conclusion, nanostructured contrast agents have been reported to be capable of making PA imaging an efficient tool for the noninvasive visualization of superficial and deep tissues. The appropriate contrast agent should be chosen for each application according to its optical properties, biocompatibility and photostability, and it can be engineered in a large variety of ways to couple PA imaging to other imaging modalities. Contrast agents for PA imaging can often serve as PTT agents, thus representing good candidates as theranostic platforms against cancer. However, more concern should be directed towards detailed and representative biocompatibility studies not only for each class of contrast agents, but for each single nanosystem. Parameters such as particle size, shape, surface charge, coating and roughness are key parameters for determining the toxicity mechanism of nanomaterials and their path in complex biosystems. For example, preliminary pharmacokinetics studies reveal that spherical gold nanoparticles can cause cell division dysfunctions while cylindrical gold nanorods are related to chronic inflammation.235 Nowadays, the main limitations concerning contrast media for PA imaging are related to the long-term toxicity and biodistribution of the nanostructures after administration and their stability upon prolonged irradiation.Thus, detailed studies are yet to be conducted, but preliminary studies confirm the promising features of several nanostructures for in vivo applications. Moreover, contrast-based PA imaging could be employed in the near future for clinical trials on humans, highlighting the need for contrast agents to achieve deeper tissue penetration and higher photostability.
In perspective, by analysing the market trend in developing novel contrast agents, which is continuously growing, and in consideration of the fact that PA is an easy, portable, low cost imaging technique that can be coupled with commonly and largely distributed echographic instrumentation, a future increase of PA applications can be expected together with an improvement in contrast agent development, specificity and applicability.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received funding from H2020 FETOPEN-2016-2017 (no. 801126, EDIT project 236–238).Notes and references
- T. N. Erpelding, A. Garcia-Uribe, A. Krumholz, H. Ke, K. Maslov, C. Appleton, J. Margenthaler and L. V. Wang, Photons Plus Ultrasound: Imaging and Sensing, 2014, 8943, 894359 Search PubMed.
- M. Xu and L. V. Wang, Rev. Sci. Instrum., 2006, 77, 041101 CrossRef.
- J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639–650 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- Y. Lao, D. Xing, S. Yang and L. Xiang, Phys. Med. Biol., 2008, 53, 4203–4212 CrossRef PubMed.
- L. J. Rich and M. Seshadri, Radiology, 2015, 275, 110–118 CrossRef PubMed.
- Q. Shao, E. Morgounova, C. Jiang, J. Choi, J. Bischof and S. Ashkenazi, J. Biomed. Opt., 2013, 18, 076019 CrossRef PubMed.
- M. Gerling, Y. Zhao, S. Nania, K. J. Norberg, C. S. Verbeke, B. Englert, R. V. Kuiper, Å. Bergström, M. Hassan, A. Neesse, J. M. Löhr and R. L. Heuchel, Theranostics, 2014, 4, 604–613 CrossRef PubMed.
- S. Mallidi, K. Watanabe, D. Timerman, D. Schoenfeld and T. Hasan, Theranostics, 2015, 5, 289–301 CrossRef PubMed.
- T. J. Allen, A. Hall, A. P. Dhillon, J. S. Owen and P. C. Beard, J. Biomed. Opt., 2012, 17, 061209 CrossRef PubMed.
- J. A. Guggenheim, T. J. Allen, A. Plumb, E. Z. Zhang, M. Rodriguez-Justo, S. Punwani and P. C. Beard, J. Biomed. Opt., 2015, 20, 050504 CrossRef PubMed.
- I. Stoffels, S. Morscher, I. Helfrich, U. Hillen, J. Leyh, N. C. Burton, T. C. P. Sardella, J. Claussen, T. D. Poeppel, H. S. Bachmann, A. Roesch, K. Griewank, D. Schadendorf, M. Gunzer and J. Klode, Sci. Transl. Med., 2015, 7, 317ra199 CrossRef PubMed.
- Q. Fan, K. Cheng, X. Hu, X. Ma, R. Zhang, M. Yang, X. Lu, L. Xing, W. Huang, S. S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 15185–15194 CrossRef CAS PubMed.
- P. Wang, P. Wang, H.-W. Wang and J.-X. Cheng, J. Biomed. Opt., 2012, 17, 0960101 CrossRef PubMed.
- P. Wang, J. R. Rajian and J. X. Cheng, J. Phys. Chem. Lett., 2013, 4, 2177–2185 CrossRef CAS PubMed.
- Gd. View Res., 2018, 1–8.
- R. E. Borg and J. Rochford, Photochem. Photobiol., 2018, 94, 1175–1209 CrossRef CAS PubMed.
- C. Brückner, Photochem. Photobiol., 2018, 1–3 Search PubMed.
- R. C. Benson and H. A. Kues, Phys. Med. Biol., 1978, 23, 017 CrossRef.
- W. M. Kuebler, J. Appl. Physiol., 2008, 104, 905–906 CrossRef PubMed.
- Z. Sheng, D. Hu, M. Xue, M. He, P. Gong and L. Cai, Nano-Micro Lett., 2013, 5, 145–150 CrossRef.
- X. Wang, G. Ku, M. A. Wegiel, D. J. Bornhop, G. Stoica and L. V. Wang, Opt. Lett., 2004, 29, 730–732 CrossRef PubMed.
- A. Miyata, T. Ishizawa, M. Kamiya, A. Shimizu, J. Kaneko, H. Ijichi, J. Shibahara, M. Fukayama, Y. Midorikawa, Y. Urano and N. Kokudo, PLoS One, 2014, 9, e112667 CrossRef PubMed.
- K. Kanazaki, K. Sano, A. Makino, T. Homma, M. Ono and H. Saji, Sci. Rep., 2016, 6, 33798 CrossRef CAS PubMed.
- C. Lutzweiler, R. Meier, E. Rummeny, V. Ntziachristos and D. Razansky, Opt. Lett., 2014, 39, 4061–4064 CrossRef PubMed.
- K. Sano, M. Ohashi, K. Kanazaki, N. Ding, J. Deguchi, Y. Kanada, M. Ono and H. Saji, Biochem. Biophys. Res. Commun., 2015, 464, 820–825 CrossRef CAS PubMed.
- J. P. Tardivo, A. Del Giglio, C. S. De Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, R. De Fatima Turchiello and M. S. Baptista, Photodiagn. Photodyn. Ther., 2005, 2, 175–191 CrossRef CAS PubMed.
- K. H. Song, E. W. Stein, J. A. Margenthaler and L. V. Wang, J. Biomed. Opt., 2008, 13, 054033 CrossRef PubMed.
- C. Kim, M. Jeon and L. V. Wang, Opt. Lett., 2011, 36, 3599 CrossRef CAS PubMed.
- W. J. Akers, W. B. Edwards, C. Kim, B. Xu, T. N. Erpelding, L. V. Wang and S. Achilefu, Transl. Res., 2012, 159, 175–181 CrossRef PubMed.
- U. Mayerhöffer, B. Fimmel and F. Würthner, Angew. Chem., Int. Ed., 2012, 51, 164–167 CrossRef PubMed.
- Y. Y. Zhang, M. Jeon, L. J. Rich, H. Hong, J. Geng, Y. Y. Zhang, S. Shi, T. E. Barnhart, P. Alexandridis, J. D. Huizinga, M. Seshadri, W. Cai, C. Kim and J. F. Lovell, Nat. Nanotechnol., 2014, 9, 631–638 CrossRef CAS PubMed.
- C. Lee, J. Kim, Y. Zhang, M. Jeon, C. Liu, L. Song, J. F. Lovell and C. Kim, Biomaterials, 2015, 73, 142–148 CrossRef CAS PubMed.
- C. J. H. Ho, G. Balasundaram, W. Driessen, R. McLaren, C. L. Wong, U. S. Dinish, A. B. E. Attia, V. Ntziachristos and M. Olivo, Sci. Rep., 2015, 4, 5342 CrossRef PubMed.
- A. Ray, X. Wang, Y. E. K. Lee, H. J. Hah, G. Kim, T. Chen, D. A. Orringer, O. Sagher, X. Liu and R. Kopelman, Nano Res., 2011, 4, 1163–1173 CrossRef PubMed.
- C. Yin, Y. Tang, X. Li, Z. Yang, J. Li, X. Li, W. Huang and Q. Fan, Small, 2018, 14, 1703400 CrossRef PubMed.
- W. J. Akers, C. Kim, M. Berezin, K. Guo, R. Fuhrhop, G. M. Lanza, G. M. Fischer, E. Daltrozzo, A. Zumbusch, X. Cai, L. V. Wang and S. Achilefu, ACS Nano, 2011, 5, 173–182 CrossRef CAS PubMed.
- Y. Ni, R. K. Kannadorai, J. Peng, S. W.-K. Yu, Y.-T. Chang and J. Wu, Chem. Commun., 2016, 52, 11504–11507 RSC.
- E. Huynh, J. F. Lovell, B. L. Helfield, M. Jeon, C. Kim, D. E. Goertz, B. C. Wilson and G. Zheng, J. Am. Chem. Soc., 2012, 134, 16464–16467 CrossRef CAS PubMed.
- X. Liang, Z. Deng, L. Jing, X. Li, Z. Dai, C. Li and M. Huang, Chem. Commun., 2013, 49, 11029 RSC.
- S. Shi, Y. Liu, Y. Chen, Z. Zhang, Y. Ding, Z. Wu and J. Yin, Theranostics, 2016, 6(12), 2170–2182 CrossRef CAS PubMed.
- D. Zhang, Y.-X. Zhao, Z.-Y. Qiao, U. Mayerhöffer, P. Spenst, X.-J. Li, F. Würthner and H. Wang, Bioconjugate Chem., 2014, 25, 2021–2029 CrossRef CAS PubMed.
- S. Bellinger, M. Hatamimoslehabadi, R. E. Borg, J. La, P. Catsoulis, F. Mithila, C. Yelleswarapu and J. Rochford, Chem. Commun., 2018, 54, 6352–6355 RSC.
- Y. Jiang and K. Pu, Adv. Biosyst., 2018, 2, 1700262 CrossRef.
- A. L. Doiron, K. A. Homan, S. Emelianov and L. Brannon-Peppas, Pharm. Res., 2009, 26, 674–682 CrossRef CAS PubMed.
- Y. Kohl, C. Kaiser, W. Bost, F. Stracke, M. Fournelle, C. Wischke, H. Thielecke, A. Lendlein, K. Kratz and R. Lemor, Nanomedicine, 2011, 7, 228–237 CrossRef CAS PubMed.
- J. P. Thawani, A. Amirshaghaghi, L. Yan, J. M. Stein, J. Liu and A. Tsourkas, Small, 2017, 13, 1–9 CrossRef PubMed.
- N. Beziere, N. Lozano, A. Nunes, J. Salichs, D. Queiros, K. Kostarelos and V. Ntziachristos, Biomaterials, 2015, 37, 415–424 CrossRef CAS PubMed.
- A. Hannah, G. Luke, K. Wilson, K. Homan and S. Emelianov, ACS Nano, 2014, 8, 250–259 CrossRef CAS PubMed.
- H. Wang, C. Liu, X. Gong, D. Hu, R. Lin, Z. Sheng, C. Zheng, M. Yan, J. Chen, L. Cai and L. Song, Nanoscale, 2014, 6, 14270–14279 RSC.
- G. Kim, S.-W. Huang, K. C. Day, M. O'Donnell, R. R. Agayan, M. A. Day, R. Kopelman and S. Ashkenazi, J. Biomed. Opt., 2007, 12, 044020 CrossRef PubMed.
- H.-W. An, S.-L. Qiao, C.-Y. Hou, Y.-X. Lin, L.-L. Li, H.-Y. Xie, Y. Wang, L. Wang and H. Wang, Chem. Commun., 2015, 51, 13488–13491 RSC.
- H. D. Lu, B. K. Wilson, A. Heinmiller, B. Faenza, S. Hejazi and R. K. Prud'homme, Appl. Mater. Interfaces, 2016, 8, 14379–14388 CrossRef PubMed.
- Q. Chen, X. Liu, J. Chen, J. Zeng, Z. Cheng and Z. Liu, Adv. Mater., 2015, 27, 6820–6827 CrossRef CAS PubMed.
- L. Zhang, S. Gao, F. Zhang, K. Yang, Q. Ma and L. Zhu, ACS Nano, 2014, 8, 12250–12258 CrossRef CAS PubMed.
- L. Xi, S. R. Grobmyer, G. Zhou, W. Qian, L. Yang and H. Jiang, J. Biophotonics, 2014, 7, 401–409 CrossRef CAS PubMed.
- L. Jing, X. Liang, Z. Deng, S. Feng, X. Li, M. Huang, C. Li and Z. Dai, Biomaterials, 2014, 35, 5814–5821 CrossRef CAS PubMed.
- Q. Mu, G. Su, L. Li, B. O. Gilbertson, L. H. Yu, Q. Zhang, Y. P. Sun and B. Yan, ACS Appl. Mater. Interfaces, 2012, 4, 2259–2266 CrossRef CAS PubMed.
- A. R. Gliga, S. Skoglund, I. Odnevall Wallinder, B. Fadeel and H. L. Karlsson, Part. Fibre Toxicol., 2014, 11, 1–17 CrossRef PubMed.
- F. G. Shellock and E. Kanal, J. Magn. Reson. Imaging, 1999, 10, 477–484 CrossRef CAS PubMed.
- D. Wu, L. Huang, M. S. Jiang and H. Jiang, Int. J. Mol. Sci., 2014, 15, 23616–23639 CrossRef PubMed.
- A. A. Oraevsky, Photoacoustics, 2015, 3, 1–2 CrossRef PubMed.
- P. Padmanabhan, A. Kumar, S. Kumar, R. K. Chaudhary and B. Gulyás, Acta Biomater., 2016, 41, 1–16 CrossRef CAS PubMed.
- M. Rai, A. P. Ingle, S. Birla, A. Yadav and C. A. Dos Santos, Crit. Rev. Microbiol., 2015, 1–24 CrossRef PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- C. L. Nehl and J. H. Hafner, J. Mater. Chem., 2008, 18, 2415 RSC.
- W. Li and X. Chen, Nanomedicine, 2015, 10, 299–320 CrossRef CAS PubMed.
- D. L. Kokkin, R. Zhang, T. C. Steimle, I. A. Wyse, B. W. Pearlman and T. D. Varberg, J. Phys. Chem. A, 2015, 119, 11659–11667 CrossRef CAS PubMed.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Chem. Soc. Rev., 2010, 39, 1805 RSC.
- N. R. Panyala, E. M. Peña-Méndez and J. Havel, J. Appl. Biomed., 2009, 7, 75–91 CAS.
- A. U. Borwankar, B. W. Willsey, A. Twu, J. J. Hung, R. J. Stover, T. W. Wang, M. D. Feldman, T. E. Milner, T. M. Truskett and K. P. Johnston, RSC Adv., 2015, 5, 104674–104687 RSC.
- H. Deng, Y. Zhong, M. Du, Q. Liu, Z. Fan, F. Dai and X. Zhang, Theranostics, 2014, 4, 904–918 CrossRef CAS PubMed.
- B. S. Gutrath, M. F. Beckmann, A. Buchkremer, T. Eckert, J. Timper, A. Leifert, W. Richtering, G. Schmitz and U. Simon, Nanotechnology, 2012, 23, 225707 CrossRef PubMed.
- R. Cheheltani, R. M. Ezzibdeh, P. Chhour, K. Pulaparthi, J. Kim, M. Jurcova, J. C. Hsu, C. Blundell, H. I. Litt, V. A. Ferrari, H. R. Allcock, C. M. Sehgal and D. P. Cormode, Biomaterials, 2016, 102, 87–97 CrossRef CAS PubMed.
- P. Huang, J. Lin, W. Li, P. Rong, Z. Wang, S. Wang, X. Wang, X. Sun, M. Aronova, G. Niu, R. D. Leapman, Z. Nie and X. Chen, Angew. Chem., Int. Ed., 2013, 52, 13958–13964 CrossRef CAS PubMed.
- J. Song, J. Kim, S. Hwang, M. Jeon, S. Jeong, C. Kim and S. Kim, Chem. Commun., 2016, 52, 8287–8290 RSC.
- T. Repenko, A. Rix, A. Nedilko, J. Rose, A. Hermann, R. Vinokur, S. Moli, R. Cao-Milàn, M. Mayer, G. von Plessen, A. Fery, L. De Laporte, W. Lederle, D. N. Chigrin and A. J. C. Kuehne, Adv. Funct. Mater., 2018, 28, 1–8 CrossRef.
- Yu. S.-S. Chang, C.-L. Lee and C. R. C. Wang, J. Phys. Chem. B, 1997, 101, 6661–6664 CrossRef.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed.
- X. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880–4910 CrossRef CAS PubMed.
- H. Chen, L. Shao, Q. Li and J. Wang, Chem. Soc. Rev., 2013, 42, 2679–2724 RSC.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 10531–10532 CrossRef CAS.
- S. Manohar, C. Ungureanu and T. G. Van Leeuwen, Contrast Media Mol. Imaging, 2011, 6, 389–400 CrossRef CAS PubMed.
- H. Qin, T. Zhou, S. Yang, Q. Chen and D. Xing, Nanomedicine, 2013, 8, 1611–1624 CrossRef CAS PubMed.
- H. W. Yang, H. L. Liu, M. L. Li, I. W. Hsi, C. T. Fan, C. Y. Huang, Y. J. Lu, M. Y. Hua, H. Y. Chou, J. W. Liaw, C. C. M. Ma and K. C. Wei, Biomaterials, 2013, 34, 5651–5660 CrossRef CAS PubMed.
- F. Gao, L. Bai, S. Liu, R. Zhang, J. Zhang, X. Feng, Y. Zheng, Y. Zhao, Y. Zhao, Y. Ma, L. Cai, Z. Cheng, H. Dai, B. T. Khuri-Yakub and S. S. Gambhir, Nanoscale, 2017, 9, 79–86 RSC.
- J. Song, X. Yang, O. Jacobson, P. Huang, X. Sun, L. Lin, X. Yan, G. Niu, Q. Ma and X. Chen, Adv. Mater., 2015, 27, 4910–4917 CrossRef CAS PubMed.
- X. Tong, Z. Wang, X. Sun, J. Song, O. Jacobson, G. Niu, D. O. Kiesewetter and X. Chen, Theranostics, 2016, 6, 2039–2051 CrossRef CAS PubMed.
- A. Agarwal, S. W. Huang, M. O'Donnell, K. C. Day, M. Day, N. Kotov and S. Ashkenazi, J. Appl. Phys., 2007, 102, 064701 CrossRef.
- K. Kim, S.-W. Huang, S. Ashkenazi, M. O'Donnell, A. Agarwal, N. A. Kotov, M. F. Denny and M. J. Kaplan, Appl. Phys. Lett., 2007, 90, 223901 CrossRef.
- M. Eghtedari, A. Oraevsky, J. A. Copland, N. A. Kotov, A. Conjusteau and M. Motamedi, Nano Lett., 2007, 7, 1914–1918 CrossRef CAS PubMed.
- J. W. M. Chon, C. Bullen, P. Zijlstra and M. Gu, Adv. Funct. Mater., 2007, 17, 875–880 CrossRef CAS.
- J. Comenge, O. Fragueiro, J. Sharkey, A. Taylor, M. Held, N. C. Burton, B. K. Park, B. Wilm, P. Murray, M. Brust and R. Lévy, ACS Nano, 2016, 10, 7106–7116 CrossRef CAS PubMed.
- Y. S. Chen, W. Frey, S. Kim, P. Kruizinga, K. Homan and S. Emelianov, Nano Lett., 2011, 11, 348–354 CrossRef CAS PubMed.
- Z. Zhang, L. Wang, J. Wang, X. Jiang, X. Li, Z. Hu, Y. Ji, X. Wu and C. Chen, Adv. Mater., 2012, 24, 1418–1423 CrossRef CAS PubMed.
- S. J. Oldenburg, J. B. Jackson, S. L. Westcott and N. J. Halas, Appl. Phys. Lett., 1999, 75, 2897–2899 CrossRef CAS.
- T. C. Preston and R. Signorell, ACS Nano, 2009, 3, 3696–3706 CrossRef CAS PubMed.
- L. Rouleau, R. Berti, V. W. K. Ng, C. Matteau-Pelletier, T. Lam, P. Saboural, A. K. Kakkar, F. Lesage, E. Rhéaume and J. C. Tardif, Contrast Media Mol. Imaging, 2013, 8, 27–39 CrossRef CAS PubMed.
- E. FarrokhTakin, G. Ciofani, G. L. Puleo, G. de Vito, C. Filippeschi, B. Mazzolai, V. Piazza and V. Mattoli, Int. J. Nanomed., 2013, 8, 2319–2331 Search PubMed.
- J. Kim, S. Park, J. E. Lee, S. M. Jin, J. H. Lee, I. S. Lee, I. Yang, J.-S. Kim, S. K. Kim, M.-H. Cho and T. Hyeon, Angew. Chem., 2006, 118, 7918–7922 CrossRef.
- J. E. Millstone, S. Park, K. L. Shuford, L. Qin, G. C. Schatz and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 5312–5313 CrossRef CAS PubMed.
- J. E. Millstone, G. S. Métraux and C. A. Mirkin, Adv. Funct. Mater., 2006, 16, 1209–1214 CrossRef CAS.
- C. Bao, N. Beziere, P. Del Pino, B. Pelaz, G. Estrada, F. Tian, V. Ntziachristos, J. M. De La Fuente and D. Cui, Small, 2013, 9, 68–74 CrossRef CAS PubMed.
- G. P. Luke, A. Bashyam, K. a. Homan, S. Makhija, Y.-S. Chen and S. Y. Emelianov, Nanotechnology, 2013, 24, 455101 CrossRef PubMed.
- J. Zhang, F. Xia, Y. Yang, C. Yue, C. Zhang, Y. Yang, L. Ma, G. Alfranca, Y. Liu, Y. Hou, W. Jin, J. Ni, J. M. de la Fuente and D. Cui, Nano Biomed. Eng., 2016, 8, 285 Search PubMed.
- X. Yang, S. E. Skrabalak, Z. Li, Y. Xia and L. V. Wang, Nano Lett., 2007, 7, 3798–3802 CrossRef CAS PubMed.
- G. D. Moon, S.-W. Choi, X. Cai, W. Li, E. C. Cho, U. Jeong, L. V. Wang and Y. Xia, J. Am. Chem. Soc., 2011, 133, 4762–4765 CrossRef CAS PubMed.
- W. Li, P. K. Brown, L. V. Wang and Y. Xia, Contrast Media Mol. Imaging, 2011, 6, 370–377 CrossRef CAS PubMed.
- J. Chen, M. Yang, Q. Zhang, E. C. Cho, C. M. Cobley, C. Kim, C. Glaus, L. V. Wang, M. J. Welch and Y. Xia, Adv. Funct. Mater., 2010, 20, 3684–3694 CrossRef CAS.
- E. C. Cho, C. Kim, F. Zhou, C. M. Cobley, K. H. Song, J. Chen, Z. Y. Li, L. V. Wang and Y. Xia, J. Phys. Chem. C, 2009, 113, 9023–9028 CrossRef CAS PubMed.
- K. H. Song, C. Kim, C. M. Cobley, Y. Xia and L. V. Wang, Nano Lett., 2009, 9, 183–188 CrossRef CAS PubMed.
- C. L. Nehl, H. Liao and J. H. Hafner, Nano Lett., 2006, 6, 683–688 CrossRef CAS PubMed.
- P. Pallavicini, A. Donà, A. Casu, G. Chirico, M. Collini, G. Dacarro, A. Falqui, C. Milanese, L. Sironi and A. Taglietti, Chem. Commun., 2013, 49, 6265 RSC.
- A. A. Umar and M. Oyama, Cryst. Growth Des., 2009, 9, 1146–1152 CrossRef.
- B. Lim and Y. Xia, Angew. Chem., 2011, 50, 76–85 CrossRef CAS PubMed.
- M. Hajfathalian, A. Amirshaghaghi, P. C. Naha, P. Chhour, J. C. Hsu, K. Douglas, Y. Dong, C. M. Sehgal, A. Tsourkas, S. Neretina and D. P. Cormode, Nanoscale, 2018, 10, 18749–18757 RSC.
- C. Pohling, J. L. Campbell, T. A. Larson, D. Van de Sompel, J. Levi, M. H. Bachmann, S. E. Bohndiek, J. V. Jokerst and S. S. Gambhir, Small, 2018, 14, 1–10 CrossRef PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS PubMed.
- W. R. Algar, K. Susumu, J. B. Delehanty and I. L. Medintz, Anal. Chem., 2011, 83, 8826–8837 CrossRef CAS PubMed.
- B. Dubertret, P. Skourides, D. J. Norris, V. Noireaux, A. H. Brivanlou and A. Libchaber, Science, 2002, 298, 1759–1762 CrossRef CAS PubMed.
- J. Mou, P. Li, C. Liu, H. Xu, L. Song, J. Wang, K. Zhang, Y. Chen, J. Shi and H. Chen, Small, 2015, 11, 2275–2283 CrossRef CAS PubMed.
- K. Yang, L. Zhu, L. Nie, X. Sun, L. Cheng, C. Wu, G. Niu, X. Chen and Z. Liu, Theranostics, 2014, 4, 134–141 CrossRef CAS PubMed.
- E. V. Shashkov, M. Everts, E. I. Galanzha and V. P. Zharov, Nano Lett., 2008, 8, 3953–3958 CrossRef CAS PubMed.
- X. Liu, W. C. Law, M. Jeon, X. Wang, M. Liu, C. Kim, P. N. Prasad and M. T. Swihart, Adv. Healthcare Mater., 2013, 2, 952–957 CrossRef CAS PubMed.
- W. Li, P. Rong, K. Yang, P. Huang, K. Sun and X. Chen, Biomaterials, 2015, 45, 18–26 CrossRef CAS PubMed.
- L. Bouchard, M. S. Anwar, G. L. Liu, B. Hann, Z. H. Xie, J. W. Gray, X. Wang, A. Pines and F. F. Chen, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4085–4089 CrossRef CAS PubMed.
- G. Ku, M. Zhou, S. Song, Q. Huang, J. Hazle and C. Li, ACS Nano, 2012, 6, 7489–7496 CrossRef CAS PubMed.
- L. M. Nie, M. Chen, X. L. Sun, P. F. Rong, N. F. Zheng and X. Y. Chen, Nanoscale, 2014, 6, 1271–1276 RSC.
- A. Ray, A. Mukundan, Z. Xie, L. Karamchand, X. Wang and R. Kopelman, Nanotechnology, 2014, 25, 445104 CrossRef PubMed.
- X. Qian, S. Shen, T. Liu, L. Cheng and Z. Liu, Nanoscale, 2015, 7, 6380–6387 RSC.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886–1893 CrossRef CAS PubMed.
- I.-T. Ho, J. L. Sessler, S. S. Gambhir and J. V. Jokerst, Analyst, 2015, 140, 3731–3737 RSC.
- B. Wu, S. T. Lu, H. Yu, R. F. Liao, H. Li, B. V. Lucie Zafitatsimo, Y. S. Li, Y. Zhang, X. L. Zhu, H. G. Liu, H. B. Xu, S. W. Huang and Z. Cheng, Biomaterials, 2018, 159, 37–47 CrossRef CAS PubMed.
- G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed.
- D. Kim, M. Lee, Y. D. Suh and S. K. Kim, J. Am. Chem. Soc., 1992, 114, 4429–4430 CrossRef CAS.
- V. Krishna, N. Stevens, B. Koopman and B. Moudgil, Nat. Nanotechnol., 2010, 5, 330–334 CrossRef CAS PubMed.
- P. W. Barone, S. Baik, D. A. Heller and M. S. Strano, Nat. Mater., 2004, 4, 86–92 CrossRef PubMed.
- M. Pramanik, M. Swierczewska, D. Green, B. Sitharaman and L. V. Wang, J. Biomed. Opt., 2009, 14, 034018 CrossRef PubMed.
- M. Pramanik, K. H. Song, M. Swierczewska, D. Green, B. Sitharaman and L. V. Wang, Phys. Med. Biol., 2009, 54, 3291–3301 CrossRef PubMed.
- F. Zhou, S. Wu, Y. Yuan, W. R. Chen and D. Xing, Small, 2012, 8, 1543–1550 CrossRef CAS PubMed.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. Sanchez Casalongue, D. Vinh and H. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- J. Shang, L. Ma, J. Li, W. Ai, T. Yu and G. G. Gurzadyan, Sci. Rep., 2012, 2, 1–8 Search PubMed.
- M. A. Patel, H. Yang, P. L. Chiu, D. D. T. Mastrogiovanni, C. R. Flach, K. Savaram, L. Gomez, A. Hemnarine, R. Mendelsohn, E. Garfunkel, H. Jiang and H. He, ACS Nano, 2013, 7, 8147–8157 CrossRef CAS PubMed.
- Z. Sheng, L. Song, J. Zheng, D. Hu, M. He, M. Zheng, G. Gao, P. Gong, P. Zhang, Y. Ma and L. Cai, Biomaterials, 2013, 34, 5236–5243 CrossRef CAS PubMed.
- G. Lalwani, X. Cai, L. Nie, L. V. Wang and B. Sitharaman, Photoacoustics, 2013, 1, 62–67 CrossRef PubMed.
- K. Yang, L. Hu, X. Ma, S. Ye, L. Cheng, X. Shi, C. Li, Y. Li and Z. Liu, Adv. Mater., 2012, 24, 1868–1872 CrossRef CAS PubMed.
- V. N. Mochalin, O. Shenderova, D. Ho and Y. Gogotsi, Nat. Nanotechnol., 2012, 7, 11–23 CrossRef CAS PubMed.
- S. Zanganeh, H. Li, P. D. Kumavor, U. Alqasemi, A. Aguirre, I. Mohammad, C. Stanford, M. B. Smith and Q. Zhu, J. Biomed. Opt., 2013, 18, 096006 CrossRef PubMed.
- G. Wang, F. Zhang, R. Tian, L. Zhang, G. Fu, L. Yang and L. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 5608–5617 CrossRef CAS PubMed.
- J. Koo, M. Jeon, Y. Oh, H. W. Kang, J. Kim, C. Kim and J. Oh, Phys. Med. Biol., 2012, 57, 7853–7862 CrossRef PubMed.
- Y.-W. Wang, Y.-Y. Fu, Q. Peng, S.-S. Guo, G. Liu, J. Li, H.-H. Yang and G.-N. Chen, J. Mater. Chem. B, 2013, 1, 5762–5767 RSC.
- K. S. Soppimath, T. M. Aminabhavi, A. R. Kulkarni and W. E. Rudzinski, J. Controlled Release, 2001, 70, 1–20 CrossRef CAS PubMed.
- C. Xie, X. Zhen, Y. Lyu and K. Pu, Adv. Mater., 2017, 29, 1–7 Search PubMed.
- A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354–2371 RSC.
- D. Zhang, M. Wu, Y. Zeng, N. Liao, Z. Cai, G. Liu, X. Liu and J. Liu, J. Mater. Chem. B, 2016, 4, 589–599 RSC.
- Y. Lyu, Y. Fang, Q. Miao, X. Zhen, D. Ding and K. Pu, ACS Nano, 2016, 10, 4472–4481 CrossRef CAS PubMed.
- D. Tuncel and H. V. Demir, Nanoscale, 2010, 2, 484–494 RSC.
- G. Balasundaram, C. J. H. Ho, K. Li, W. Driessen, U. S. Dinish, C. L. Wong, V. Ntziachristos, B. Liu and M. Olivo, Int. J. Nanomed., 2015, 10, 387–397 CrossRef PubMed.
- Z. Zha, Z. Deng, Y. Li, C. Li, J. Wang, S. Wang, E. Qu and Z. Dai, Nanoscale, 2013, 5, 4462 RSC.
- T. Stahl, R. Bofinger, I. Lam, K. J. Fallon, P. Johnson, O. Ogunlade, V. Vassileva, R. B. Pedley, P. C. Beard, H. C. Hailes, H. Bronstein and A. B. Tabor, Bioconjugate Chem., 2017, 28, 1734–1740 CrossRef CAS PubMed.
- X. Liang, Y. Li, X. Li, L. Jing, Z. Deng, X. Yue, C. Li and Z. Dai, Adv. Funct. Mater., 2015, 25, 1451–1462 CrossRef CAS.
- C. Xie, P. K. Upputuri, X. Zhen, M. Pramanik and K. Pu, Biomaterials, 2017, 119, 1–8 CrossRef CAS PubMed.
- T. Repenko, S. Fokong, L. De Laporte, D. Go, F. Kiessling, T. Lammers and A. J. C. Kuehne, Chem. Commun., 2015, 51, 6084–6087 RSC.
- X. Cai, X. Liu, L. De Liao, A. Bandla, J. M. Ling, Y. H. Liu, N. Thakor, G. C. Bazan and B. Liu, Small, 2016, 12, 4873–4880 CrossRef CAS PubMed.
- D. Pan, X. Cai, B. Kim, A. J. Stacy, L. V. Wang and G. M. Lanza, Adv. Healthcare Mater., 2012, 1, 582–589 CrossRef CAS PubMed.
- C. Yin, X. Zhen, Q. Fan, W. Huang and K. Pu, ACS Nano, 2017, 11, 4174–4182 CrossRef CAS PubMed.
- X. Zhen, C. Xie, Y. Jiang, X. Ai, B. Xing and K. Pu, Nano Lett., 2018, 18, 1498–1505 CrossRef CAS PubMed.
- C. Xie, X. Zhen, Q. Lei, R. Ni and K. Pu, Adv. Funct. Mater., 2017, 27, 1605397 CrossRef.
- K. Shou, Y. Tang, H. Chen, S. Chen, L. Zhang, A. Zhang, Q. Fan, A. Yu and Z. Cheng, Chem. Sci., 2018, 9, 3105–3110 RSC.
- M. Jeon, W. Song, E. Huynh, J. Kim, J. Kim, B. L. Helfield, B. Y. C. Leung, D. E. Goertz, G. Zheng, J. Oh, J. F. Lovell and C. Kim, J. Biomed. Opt., 2014, 8943, 894321 Search PubMed.
- J. Zhong, S. Yang, X. Zheng, T. Zhou and D. Xing, Nanomedicine, 2013, 8, 903–919 CrossRef CAS PubMed.
- K. Miki, T. Inoue, Y. Kobayashi, K. Nakano, H. Matsuoka, F. Yamauchi, T. Yano and K. Ohe, Biomacromolecules, 2015, 16, 219–227 CrossRef CAS PubMed.
- E. Swider, K. Daoudi, A. H. J. Staal, O. Koshkina, N. Koen Van Riessen, E. Van Dinther, I. Jolanda, M. De Vries, C. L. De Korte and M. Srinivas, Nanotheranostics, 2018, 2, 258–268 CrossRef PubMed.
- S. Sreejith, J. Joseph, M. Lin, N. V. Menon, P. Borah, H. J. Ng, Y. X. Loong, Y. Kang, S. W. K. Yu and Y. Zhao, ACS Nano, 2015, 9, 5695–5704 CrossRef CAS PubMed.
- Q. Fan, K. Cheng, Z. Yang, R. Zhang, M. Yang, X. Hu, X. Ma, L. Bu, X. Lu, X. Xiong, W. Huang, H. Zhao and Z. Cheng, Adv. Mater., 2015, 27, 843–847 CrossRef CAS PubMed.
- Z. Yang, R. Tian, J. Wu, Q. Fan, B. C. Yung, G. Niu, O. Jacobson, Z. Wang, G. Liu, G. Yu, W. Huang, J. Song and X. Chen, ACS Nano, 2017, 11, 4247–4255 CrossRef CAS PubMed.
- C. Kim, K. H. Song, F. Gao and L. V. Wang, Radiology, 2010, 255, 442–450 CrossRef PubMed.
- K. E. Wilson, S. V. Bachawal and J. K. Willmann, Clin. Cancer Res., 2018, 24, 3572–3582 CrossRef CAS PubMed.
- A. Garcia-Uribe, T. N. Erpelding, A. Krumholz, H. Ke, K. Maslov, C. Appleton, J. A. Margenthaler and L. V. Wang, Sci. Rep., 2015, 5, 15748 CrossRef CAS PubMed.
- K. Wilson, K. Homan and S. Emelianov, Nat. Commun., 2012, 3, 610–618 CrossRef PubMed.
- D. Pan, M. Pramanik, A. Senpan, S. Ghosh, S. A. Wickline, L. V. Wang and G. M. Lanza, Biomaterials, 2010, 31, 4088–4093 CrossRef CAS PubMed.
- D. Pan, M. Pramanik, S. A. Wickline, L. V. Wang and G. M. Lanza, Contrast Media Mol. Imaging, 2011, 6, 378–388 CrossRef CAS PubMed.
- M. F. Kircher, A. De La Zerda, J. V. Jokerst, C. L. Zavaleta, P. J. Kempen, E. Mittra, K. Pitter, R. Huang, C. Campos, F. Habte, R. Sinclair, C. W. Brennan, I. K. Mellinghoff, E. C. Holland and S. S. Gambhir, Nat. Med., 2012, 18, 829–834 CrossRef CAS PubMed.
- X. Cheng, R. Sun, L. Yin, Z. Chai, H. Shi and M. Gao, Adv. Mater., 2017, 29, 1604894 CrossRef PubMed.
- J. V. Jokerst, A. J. Cole, D. Van De Sompel, S. S. Gambhir, D. de Sompel, S. S. Gambhir, D. Van De Sompel and S. S. Gambhir, ACS Nano, 2012, 6, 10366–10377 CrossRef CAS PubMed.
- T. Guo, Y. Lin, Z. Li, S. Chen, G. Huang, H. Lin, J. Wang, G. Liu and H.-H. Yang, Nanoscale, 2017, 9, 56–61 RSC.
- Y. Du, Q. Jiang, N. Beziere, L. Song, Q. Zhang, D. Peng, C. Chi, X. Yang, H. Guo, G. Diot, V. Ntziachristos, B. Ding and J. Tian, Adv. Mater., 2016, 28, 10000–10007 CrossRef CAS PubMed.
- N. Yan, X. Wang, L. Lin, T. Song, P. Sun, H. Tian, H. Liang and X. Chen, Adv. Funct. Mater., 2018, 28, 1–10 Search PubMed.
- I. Monaco, F. Arena, S. Biffi, E. Locatelli, B. Bortot, F. La Cava, G. M. Marini, G. M. Severini, E. Terreno and M. Comes Franchini, Bioconjugate Chem., 2017, 28, 1382–1390 CrossRef CAS PubMed.
- M. Jeon, S. Jenkins, J. Oh, J. Kim, T. Peterson, J. Chen and C. Kim, Nanomedicine, 2014, 9, 1377–1388 CrossRef CAS PubMed.
- S. Raveendran, H. T. Lim, T. Maekawa, M. Vadakke Matham and D. Sakthi Kumar, Nanoscale, 2018, 10, 13959–13968 RSC.
- C. Kim, E. C. Cho, J. Chen, K. H. Song, L. Au, C. Favazza, Q. Zhang, C. M. Cobley, F. Gao, Y. Xia and L. V. Wang, ACS Nano, 2010, 4, 4559–4564 CrossRef CAS PubMed.
- C. Bao, J. Conde, F. Pan, C. Li, C. Zhang, F. Tian, S. Liang, J. M. de la Fuente and D. Cui, Nano Res., 2016, 9, 1043–1056 CrossRef CAS.
- J. Han, J. Zhang, M. Yang, D. Cui and J. M. de la Fuente, Nanoscale, 2016, 8, 492–499 RSC.
- K. Cheng, S. R. Kothapalli, H. Liu, A. L. Koh, J. V. Jokerst, H. Jiang, M. Yang, J. Li, J. Levi, J. C. Wu, S. S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 3560–3571 CrossRef CAS PubMed.
- S. Liang, C. Li, C. Zhang, Y. Chen, L. Xu, C. Bao, X. Wang, G. Liu, F. Zhang and D. Cui, Theranostics, 2015, 5, 970–984 CrossRef CAS PubMed.
- S. K. Maji, S. Sreejith, J. Joseph, M. Lin, T. He, Y. Tong, H. Sun, S. W. K. Yu and Y. Zhao, Adv. Mater., 2014, 26, 5633–5638 CrossRef CAS PubMed.
- Y. Sheng, L.-D. Liao, N. Thakor and M. C. Tan, Sci. Rep., 2015, 4, 6562 CrossRef PubMed.
- Z. Sun, Y. Zhao, Z. Li, H. Cui, Y. Zhou, W. Li, W. Tao, H. Zhang, H. Wang, P. K. Chu and X.-F. Yu, Small, 2017, 13, 1602896 CrossRef PubMed.
- C. Sun, L. Wen, J. Zeng, Y. Wang, Q. Sun, L. Deng, C. Zhao and Z. Li, Biomaterials, 2016, 91, 81–89 CrossRef CAS PubMed.
- G. Lv, W. Guo, W. Zhang, T. Zhang, S. Li, S. Chen, A. S. Eltahan, D. Wang, Y. Wang, J. Zhang, P. C. Wang, J. Chang and X. J. Liang, ACS Nano, 2016, 10, 9637–9645 CrossRef CAS PubMed.
- X. R. Song, X. Wang, S. X. Yu, J. Cao, S. H. Li, J. Li, G. Liu, H. H. Yang and X. Chen, Adv. Mater., 2015, 27, 3285–3291 CrossRef CAS PubMed.
- F. Gong, L. Cheng, N. Yang, Q. Jin, L. Tian, M. Wang, Y. Li and Z. Liu, Nano Lett., 2018, 18, 6037–6044 CrossRef CAS PubMed.
- D. Pan, X. Cai, C. Yalaz, A. Senpan, K. Omanakuttan, S. A. Wickline, L. V. Wang and G. M. Lanza, ACS Nano, 2012, 6, 1260–1267 CrossRef CAS PubMed.
- Z. Zhou, K. Hu, R. Ma, Y. Yan, B. Ni, Y. Zhang, L. Wen, Q. Zhang and Y. Cheng, Adv. Funct. Mater., 2016, 26, 5971–5978 CrossRef CAS.
- S. Park, G. Park, J. Kim, W. Choi, U. Jeong and C. Kim, Nanoscale, 2018, 10, 20548–20558 RSC.
- M. Chen, S. Tang, Z. Guo, X. Wang, S. Mo, X. Huang, G. Liu and N. Zheng, Adv. Mater., 2014, 26, 8210–8216 CrossRef CAS PubMed.
- K. A. Homan, M. Souza, R. Truby, G. P. Luke, C. Green, E. Vreeland and S. Emelianov, ACS Nano, 2012, 6, 641–650 CrossRef CAS PubMed.
- M. G. Cha, S. Lee, S. Park, H. Kang, S. G. Lee, C. Jeong, Y.-S. Lee, C. Kim and D. H. Jeong, Nanoscale, 2017, 12556–12564 RSC.
- W. He, K. Ai, C. Jiang, Y. Li, X. Song and L. Lu, Biomaterials, 2017, 132, 37–47 CrossRef CAS PubMed.
- U. Guler, V. M. Shalaev and A. Boltasseva, Mater. Today, 2015, 18, 227–237 CrossRef CAS.
- A. De la Zerda, C. Zavaleta, S. Keren, S. Vaithilingam, S. Bodapati, Z. Liu, J. Levi, B. R. Smith, T. J. Ma, O. Oralkan, Z. Cheng, X. Chen, H. Dai, B. T. Khuri-Yakub and S. S. Gambhir, Nat. Nanotechnol., 2008, 3, 557–562 CrossRef CAS PubMed.
- A. de la Zerda, Z. Liu, S. Bodapati, R. Teed, S. Vaithilingam, B. T. Khuri-Yakub, X. Chen, H. Dai and S. S. Gambhir, Nano Lett., 2010, 10, 2168–2172 CrossRef CAS PubMed.
- A. De La Zerda, S. Bodapati, R. Teed, S. Y. May, S. M. Tabakman, Z. Liu, B. T. Khuri-Yakub, X. Chen, H. Dai and S. S. Gambhir, ACS Nano, 2012, 6, 4694–4701 CrossRef CAS PubMed.
- D. Chen, C. Wang, X. Nie, S. Li, R. Li, M. Guan, Z. Liu, C. Chen, C. Wang, C. Shu and L. Wan, Adv. Funct. Mater., 2014, 24, 6621–6628 CrossRef CAS.
- V. Krishna, A. Singh, P. Sharma, N. Iwakuma, Q. Wang, Q. Zhang, J. Knapik, H. Jiang, S. R. Grobmyer, B. Koopman and B. Moudgil, Small, 2010, 6, 2236–2241 CrossRef CAS PubMed.
- L. Wu, X. Cai, K. Nelson, W. Xing, J. Xia, R. Zhang, A. J. Stacy, M. Luderer, G. M. Lanza, L. V. Wang, B. Shen and D. Pan, Nano Res., 2013, 6, 312–325 CrossRef CAS PubMed.
- F. Wu, H. Su, Y. Cai, W.-K. Wong, W. Jiang and X. Zhu, ACS Appl. Bio Mater., 2018, 1, 110–117 CrossRef CAS.
- T. Zhang, H. Cui, C.-Y. Fang, L.-I. Su, S. Ren, H.-C. Chang, X. Yang and M. Laird Forrest, J. Biomed. Opt., 2013, 18, 026018 CrossRef PubMed.
- K. Pu, A. J. Shuhendler, J. V. Jokerst, J. Mei, S. S. Gambhir, Z. Bao and J. Rao, Nat. Nanotechnol., 2014, 9, 233–239 CrossRef CAS PubMed.
- K. Pu, J. Mei, J. V. Jokerst, G. Hong, A. L. Antaris, N. Chattopadhyay, A. J. Shuhendler, T. Kurosawa, Y. Zhou, S. S. Gambhir, Z. Bao and J. Rao, Adv. Mater., 2015, 27, 5184–5190 CrossRef CAS.
- E. Huynh, B. Y. C. Leung, B. L. Helfield, M. Shakiba, J. A. Gandier, C. S. Jin, E. R. Master, B. C. Wilson, D. E. Goertz and G. Zheng, Nat. Nanotechnol., 2015, 10, 325–332 CrossRef CAS PubMed.
- Y. Lyu, X. Zhen, Y. Miao and K. Pu, ACS Nano, 2017, 11, 358–367 CrossRef CAS PubMed.
- Y. Jiang, P. K. Upputuri, C. Xie, Y. Lyu, L. Zhang, Q. Xiong, M. Pramanik and K. Pu, Nano Lett., 2017, 17, 4964–4969 CrossRef CAS PubMed.
- L. Li, A. A. Shemetov, M. Baloban, P. Hu, L. Zhu, D. M. Shcherbakova, R. Zhang, J. Shi, J. Yao, L. V. Wang and V. V. Verkhusha, Nat. Commun., 2018, 9, 1–14 CrossRef PubMed.
- J.-W. Kim, E. I. Galanzha, E. V. Shashkov, H.-M. Moon and V. P. Zharov, Nat. Nanotechnol., 2009, 4, 688–694 CrossRef CAS PubMed.
- X. Liu, C. Lee, W.-C. Law, D. Zhu, M. Liu, M. Jeon, J. Kim, P. N. Prasad, C. Kim and M. T. Swihart, Nano Lett., 2013, 13, 4333–4339 CrossRef CAS PubMed.
- C. Wang, C. Bao, S. Liang, H. Fu, K. Wang, M. Deng, Q. Liao and D. Cui, Nanoscale Res. Lett., 2014, 9, 264 CrossRef PubMed.
- H. Moon, H. H. Kim, D. Kumar, H. H. Kim, C. Sim, J. H. Chang, J. M. Kim and D. K. Lim, ACS Nano, 2015, 9, 2711–2719 CrossRef CAS PubMed.
- S. Gao, L. Zhang, G. Wang, K. Yang, M. Chen, R. Tian, Q. Ma and L. Zhu, Biomaterials, 2016, 79, 36–45 CrossRef CAS PubMed.
- Y.-S. Chen, S. J. Yoon, W. Frey, M. Dockery and S. Emelianov, Nat. Commun., 2017, 8, 15782 CrossRef CAS PubMed.
- I. Monaco, P. Armanetti, E. Locatelli, A. Flori, M. Maturi, S. Del Turco, L. Menichetti and M. Comes Franchini, J. Mater. Chem. B, 2018, 6, 2993–2999 RSC.
- S. Sharifi, S. Behzadi, S. Laurent, M. Laird Forrest, P. Stroeve and M. Mahmoudi, Chem. Soc. Rev., 2012, 41, 2323–2343 RSC.
- X. Liu, M. Atwater, J. Wang and Q. Huo, Colloids Surf., B, 2007, 58, 3–7 CrossRef CAS PubMed.
- K. Park, S. Biswas, S. Kanel, D. Nepal and R. A. Vaia, J. Phys. Chem. C, 2014, 118, 5918–5926 CrossRef CAS.
- F. Schöppler, C. Mann, T. C. Hain, F. M. Neubauer, G. Privitera, F. Bonaccorso, D. Chu, A. C. Ferrari and T. Hertel, J. Phys. Chem. C, 2011, 115, 14682–14686 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |