Biomaterials as vectors for the delivery of CRISPR–Cas9
Abstract
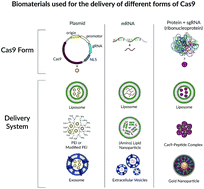
The emergence of the CRISPR–Cas9 gene editing system has brought much hope and excitement to the field of gene therapy and the larger scientific community. However, in order for CRISPR-based therapies to be translated to the clinical setting, there is an urgent need to develop optimized vectors for their delivery. The delivery vector is a crucial determinant of the therapeutic efficacy of gene editing and should be designed to accommodate various factors including the form of the payload, the physiological environment, and the potential immune responses. Recently, biomaterials have become an attractive option for the delivery of Cas9 due to their tunability, biocompatibility and increasing efficacy at drug delivery. Biomaterials offer a unique solution for creating tailored vectors to meet the demands of various applications that cannot be easily met by other delivery methods. In this review, we will discuss the various biomaterial systems that have been used to deliver Cas9 in its plasmid, mRNA and protein forms. In addition, the functions of these materials will be reviewed to understand their roles in Cas9 delivery. Finally, the immune challenges associated with Cas9 and the delivery materials will be discussed as an understanding of the immune responses along with the functions of biomaterials will ultimately guide the field in designing new delivery systems for the clinical applications of CRISPR–Cas9.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...