Printed nanofilms mechanically conforming to living bodies
Abstract
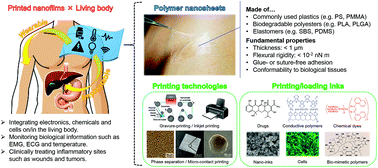
It is anticipated that flexible wearable/implantable devices for biomedical applications will be established for the development of medical diagnostics and therapeutics. However, these devices need to be compatible with the physical and mechanical properties of the living body. In this minireview, we introduce free-standing polymer ultra-thin films (referred to as “polymer nanosheets”), for which a variety of polymers can be selected as building blocks (e.g., biodegradable polymers, conductive polymers, and elastomers), as a platform for flexible biomedical devices that are mechanically compatible with the living body, and then we demonstrate the use of “printed nanofilms” by combining nanosheets and printing technologies with a variety of inks represented by drugs, conductive nanomaterials, chemical dyes, bio-mimetic polymers, and cells. Owing to the low flexural rigidity (<10−2 nN m) of the polymer nanosheets, which is within the range of living brain slices (per unit width), the flexible printed nanofilms realize bio-integrated structure and display various functions with unique inks that continually monitor or detect biological activities, such as performing surface electromyography, measuring epidermal strain, imaging tissue temperature, organizing cells, and treating lesions in wounds and tumors.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...