Adipose-derived stem cell-secreted factors promote early stage follicle development in a biomimetic matrix†
Abstract
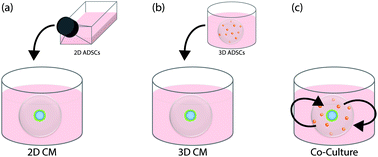
Development of primary follicles in vitro benefits from a three-dimensional matrix that is enriched with paracrine factors secreted from feeder cells and mimics the in vivo environment. In this study, we investigated the role of paracrine signaling from adipose-derived stem cells (ADSCs) in supporting primary follicle development in a biomimetic poly(ethylene glycol) (PEG)-based matrix. Follicles co-cultured with ADSCs and follicles cultured in conditioned medium from ADSCs encapsulated in gels (3D CM) exhibited significantly (p < 0.01 and p = 0.09, respectively) improved survival compared to follicles cultured in conditioned medium collected from ADSCs cultured in flasks (2D CM) and follicles cultured without paracrine support. The gene expression of ADSCs suggested that the stem cells maintained their multipotency in the 3D PEG environment over the culture period, regardless of the presence of the follicles, while under 2D conditions the multipotency markers were downregulated. The differences in cytokine signatures of follicles exposed to 3D and 2D ADSC paracrine factors suggest that early cytokine interactions are key for follicle survival. Taken together, the biomimetic PEG scaffold provides a three-dimensional, in vivo-like environment to induce ADSCs to secrete factors which promote early stage ovarian follicle development and survival.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...
