A pH-responsive hydrogel with potent antibacterial activity against both aerobic and anaerobic pathogens†
Abstract
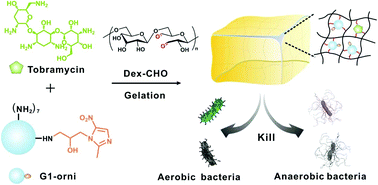
Aminoglycosides are a family of antibiotics with a wide-spread antibacterial spectrum and high antibacterial activity. However, they are less effective against anaerobic pathogens due to the requirement of aerobic respiration to exert their antibacterial functions. Here, we prepared a pH-responsive hydrogel with potent antibacterial activities against both aerobic and anaerobic pathogens. The hydrogel was prepared by reacting oxidized dextran with aminoglycoside and an ornidazole analogue via an acid-labile Schiff base linkage. The prepared hydrogel was injectable, self-healing, biocompatible, and showed a pH-responsive drug release behavior. More importantly, the gel was efficient in killing both aerobic and anaerobic pathogens. This study provides a facile strategy to expand the antibacterial spectrum of aminoglycoside hydrogels.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...