Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The role of a semi-automated NanoVelcro system in capturing circulating tumor cells and evaluating their prognostic value for gestational choriocarcinoma†
Minzhi
Hou‡
ab,
Yongjiang
Zheng‡
c,
Zhiming
Ding‡
d,
Shanyang
He
e,
Manman
Xu
c,
Xinlin
Chen‡
f,
Hui
Zhang‡
g,
Chao
Zeng
ah,
Cong
Sun
g,
Wenting
Jiang‡
a,
Han
Wang
a,
Hongwei
Shen
c,
Yang
Zhang
i,
Jing
Liu
j,
Shijun
Sun
k,
Neng
Jiang
a,
Yongmei
Cui
a,
Yu
Sun
a,
Yangshan
Chen
a,
Jessica
Cao
l,
Chunlin
Wang
m,
Mengzhen
Li
n,
Yi
Zhang
o,
Jianhong
Wang
p,
Millicent
Lin
q and
Zunfu
Ke
 *agr
*agr
aDepartment of Pathology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, P.R. China. E-mail: kezunfu@mail.sysu.edu.cn; Fax: +86-20-87331780; Tel: +86-20-87331780
bDepartment of Gynecology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, P.R. China
cDepartment of Hematology, The Third Affiliated Hospital of Sun Yat-Sen University, 600 Tianhe Road, Guangzhou, Guangdong, China
dDepartment of Neurosurgery, The Eastern Hospital of the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, P.R. China
eDepartment of Gynecology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, P.R. China
fSchool of Basic Medical Science, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, P.R. China
gInstitute of Precision Medicine, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, P.R. China
hDepartment of Pathology, Guangdong Medical College, Dongguan, Guangdong, P.R. China
iBiomedical Engineering, The University of Texas at El Paso, El Paso, TX, USA
jDepartment of Anesthesiology, Guangdong Women and Children Hospital, Guangzhou, Guangdong, P.R. China
kMolecular Diagnosis Center, The Affiliated Zhongshan Hospital, Sun Yat-Sen University, Zhongshan, Guangdong, P.R. China
lCumming School of Medicine, University of Calgary, Calgary, Alberta, Canada
mChapter Diagnostics, Menlo Park, California 94025, USA
nMyGene Diagnostics, Guangzhou International Biotech Island, Guangdong, P.R. China
oBiomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA
pPricision Medicine Center, Shenzhen People's Hospital, Shenzhen, Guangdong, P.R. China
qDepartment of Genetics, Harvard Medical School, Boston, MA, USA
rGuangdong Provincial Key Laboratory of Orthopedics and Traumatology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
First published on 16th January 2019
Abstract
To investigate whether circulating tumor cells (CTCs) are detectable in patients with gestational choriocarcinoma (GC) and evaluate the prognostic value of CTC enumeration. In this multicenter study, the presence of CTCs was examined in 180 GC patients using a semi-automated NanoVelcro system, among whom 106 patients underwent CTC re-evaluation after one cycle of chemotherapy. Approximately 96% of the GC patients contained ≥2 CTCs in 7.5 mL of blood. The number of CTCs per 7.5 mL of blood was much higher in patients with distant metastases (n = 95; range, 0 to 104) than in patients without distant metastases (n = 85; range, 0 to 6). Applying a 90-patient training and 90-patient validation cohort, a cutoff value of ≥6 CTCs was defined as the prognostic threshold for progression-free survival (PFS) and overall survival (OS). The presence of ≥6 CTCs was significantly associated with worse PFS and OS (both P < 0.001). A multivariate analysis showed that the CTC number (≥6 CTCs) was the strongest predictor of OS (hazard ratio [HR], 15.8; 95% confidence interval [CI], 4.3–57.9; P < 0.001). The number of CTCs decreased after one cycle of chemotherapy; univariate analyses demonstrated that the CTC count after the first chemotherapy cycle was a strong predictor of OS (HR, 36.1; 95% CI, 4.8–271.5; P < 0.001). CTCs are a promising prognostic factor for GC. The absolute CTC count after one cycle of chemotherapy in the context of this disease is a strong predictor of chemotherapy response.
Introduction
Gestational choriocarcinoma (GC), a type of aggressive and malignant gestational trophoblastic neoplasia, occurs in approximately 1 in 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 40
000 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 any form of previous conceptions.1 Due to the efficiency of chemotherapy and the dynamic monitoring management based on beta human chorionic gonadotropin (β-HCG), the majority of GC cases are usually curable.2,3 However, critical clinical information such as comprehensive metastasis status and resistance to conventional chemotherapy manifested in some cases cannot be fully revealed and assessed only by the β-HCG level.
000 any form of previous conceptions.1 Due to the efficiency of chemotherapy and the dynamic monitoring management based on beta human chorionic gonadotropin (β-HCG), the majority of GC cases are usually curable.2,3 However, critical clinical information such as comprehensive metastasis status and resistance to conventional chemotherapy manifested in some cases cannot be fully revealed and assessed only by the β-HCG level.
Over the years, the International Federation of Gynecology and Obstetrics (FIGO) staging and the FIGO/WHO prognostic scoring system have played a pivotal role not only in reflecting the metastasis characteristics, but also in somehow acting as important prognostic factors for GC patients.4 However, there are still some limitations in the FIGO staging and the scoring system, as they are mainly based on imaging examinations and laboratory tests, failing to recognize cases that can develop into chemoresistance. Additionally, high level β-HCG does not reflect the risk of disease and the prognosis in parallel.5 Thus, further exploration on effective clinical indicators to assist disease-status and chemotherapy response evaluation is of great significance to guide the clinical management of GC.
Circulating tumor cells (CTCs), originating from the primary or the metastasis lesions and disseminating to the peripheral blood circulation, are a source of cancer hematogenous metastasis, and show great clinical value, especially as a prognostic marker for many different cancer types.6,7 As a simple, noninvasive, and easily repeated “liquid biopsy,” evaluation of circulating tumor cells (CTCs) provides the opportunity to longitudinally monitor tumor status at different time points during therapy.8 Hence, we conducted the present study to clarify the significance of CTC counts in predicting the prognosis and evaluating the chemotherapy response in patients with GC.
Methods
Patients
Totally, 180 GC patients from multiple centers (The First Affiliated Hospital, Sun Yat-sen University, Guangdong Women and Children Hospital, The Affiliated Zhongshan Hospital etc.) were enrolled into this study from October 2009 to October 2013 (ESI Table S1†). All participants provided written informed consent. All animal procedures in this study were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Sun Yat-sen University and approved by the Animal Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (Protocol No. A-084). The patients recruited were at least 18 years of age and had been confirmed by two pathologists. Those with pregnancy simultaneously or during the treatment were excluded. Also, patients out of follow-up were excluded. FIGO staging was classified using the published staging systems for gynecological cancers in the twenty-sixth volume of the FIGO Annual Report.9 Metastatic disease was determined by medical history, physical examination, blood chemistry analysis, and ultrasonography or routine computed tomography (CT), or magnetic resonance imaging (MRI). To avoid the chemotherapy effect on the CTC count and activity, we excluded those who received any chemotherapy in the previous 3 months at the time of the first CTC test10 (ESI Fig. S1†). We performed the second CTC test (post-treatment CTC) count within 2 days after the end of the first chemotherapy cycle. Thus, the pretreatment CTC count was from the GC patients who did not receive any chemotherapy in the previous 3 months and accepted the first CTC test within one week before the first chemotherapy.CTC detection by the NanoVelcro system
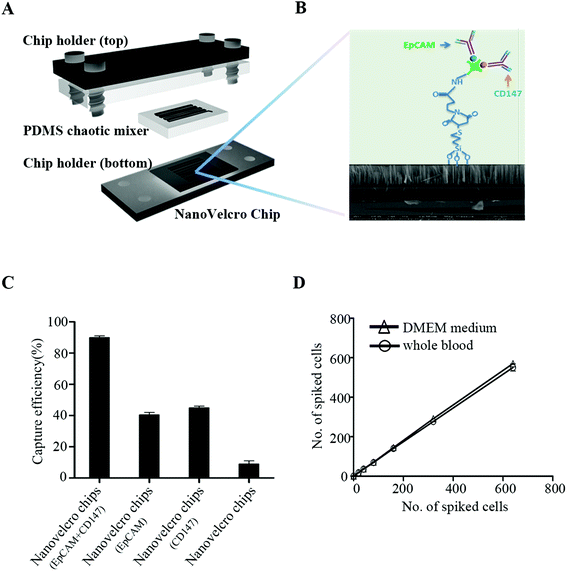
CTC enrichment was performed using the NanoVelcro (Cytolumina, Los Angeles, USA) system, as previously described.11,12 The NanoVelcro substrate was modified with a combination of EpCAM and CD147, to increase the capture efficiency of CTCs from the peripheral blood samples of GC patients (Fig. 1). The peripheral blood samples (7.5 mL) were collected from GC patients with different stages of the disease and preserved in CellSave Tubes (containing fixation agents). After being introduced into integrated devices and rinsed with PBS, fixation and permeabilization agents were put into the devices which were then incubated for 30 min. A commonly used three-color immunocytochemistry method was utilized to identify CTCs, including a TRITC-conjugated anti-CD45 antibody (Sigma, USA) and a FITC-conjugated anti-β-HCG antibody (Abcam, USA). CTCs showed strong β-HCG expression and negligible CD45 signals.Statistical analysis
Assuming a power of ≥90% and a two-sided α of 0.05, a sample size of 153 would meet the statistical requirements for detecting the difference between a median PFS of 43 months for the “favorable” CTC group and a median PFS of 21 months for the “unfavorable” CTC group. Because we were not sure of the proportion of patients randomly allocated to each group, we increased the sample size to 180 to allow for a favorable-to-unfavorable CTC group ratio as low as 0.5 or as high as 2.3. To obtain the most appropriate CTC cutoff for distinguishing prognosis, all the enrolled GC patients were randomly split into the training and validation cohorts. In the training phase, a range of baseline CTC values for 90 enrolled patients were tested to establish an optimal cutoff level. In the validation phase, the optimal cutoff level was then evaluated with new data collected from an independent cohort of 90 enrolled GC patients.The associations of CTC counts and clinicopathological characteristics were compared using the chi-square test or Fisher's exact test. Correlations between CTC counts and binary and ordinal data were analyzed using the chi-square test or Fisher's exact and Spearman's rank test respectively. Univariate Cox proportional hazards regression for both PFS and OS was performed to analyze the relevant clinical parameters, including serum β-HCG level, resistance to multiagent chemotherapy, metastatic sites, FIGO stages and baseline CTC values. Multivariable Cox regression was applied to the selected significant variables for PFS and OS using stepwise methods. Survival curves of the different CTC groups were compared using log-rank testing. Statistical analysis was performed using SPSS 13.0 for Windows (SPSS, Chicago, IL). A 2-sided P-value < 0.05 was considered statistically significant.
Results
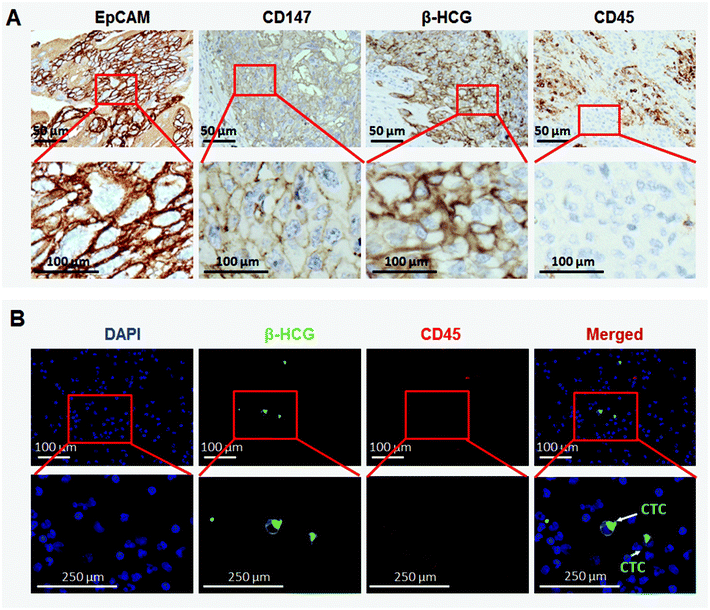
EpCAM and CD147 expression characteristics in GC tissues, and CTC enrichment
Our preliminary experiment shows that EpCAM and CD147 staining was mainly detected on the cell membrane (Fig. 2A). Among 180 GC patients, 172 patients were detected to have a positive expression of EpCAM and only 8 patients to have a negative expression. For CD147, 175 of them were found to have a positive expression and merely 5 were found with negative staining. None of them was negative for both EpCAM and CD147 (Table 1). Being a unique marker of trophoblastic disease, a strong expression of β-HCG was observed in all GC tissues. However, all GC cells exhibited negative staining for CD45, the white cell specific marker (Fig. 2A). These were consistent with other research studies,13,14 and EpCAM and CD147 were recognized as suitable markers for CTC detection and enumeration15,16 (Fig. 1). As observed, the capture efficiency of NanoVelcro chips with EpCAM + CD147 could reach ∼90%, much higher than NanoVelcro chips with EpCAM or CD147 (Fig. 1C). To evaluate the capture efficiency of NanoVelcro chips with EpCAM + CD147, different numbers of JEG-3 cells prestained with DIO, ranging from 10 to 640, were spiked into both DMEM and whole blood. Consistent recovery rates were observed at various numbers of spiked cells as low as 10 cells per mL (Fig. 1D).| EpCAM staining | n (%) | CD147 staining | |
|---|---|---|---|
| Positive (%) | Negative (%) | ||
| Positive | 172 (95.6) | 167 (92.78) | 5 (2.78) |
| Negative | 8 (4.44) | 8 (4.44) | 0 |
After capturing the suspicious CTCs, we performed immunofluorescence staining to confirm the accuracy of captured CTCs. β-HCG was found to be merely expressed in CTCs, which were negative for CD45 (Fig. 2B). This is consistent with IHC staining in GC tissues.
Defining the most appropriate prognostic cutoff for the CTC count
In order to establish the optimum CTC count cutoff, a series of CTC thresholds were systematically evaluated for their estimate of PFS and OS by the Kaplan–Meier method and log-rank test in a training set. After comparing the hazard ratios (HRs) and differences by multiple-threshold testing, a cutoff of 6 CTCs per 7.5 mL was found to offer optimal PFS and OS prediction (ESI Tables S2 and S3†). Thus, a cutoff of 6 CTCs was used thereafter to distinguish between high- and low-risk patients. The reliability of our CTC cutoff was further verified in a validation cohort. As shown in ESI Fig. S2A–F,† the cutoff of 6 CTCs per 7.5 mL for PFS and OS was fully supported by the validation set.Relationship of the pretreatment CTC count with clinicopathological characteristics
The different pretreatment CTC baseline values and their corresponding associations with clinical characteristics are listed in detail in Table 2. Based on the optimal prognostic CTC threshold (≥6 CTCs in 7.5 mL of blood), a significant association was observed between the CTC count and the FIGO score (P < 0.001) and the FIGO stage (P < 0.001). For the 35 FIGO IV patients, 30 (85.7%) had 6 CTCs in 7.5 mL of blood (range, 3 to 54). This positive rate was significantly higher than that in FIGO III and II patients, at 11.7% (7/60, range, 0 to 104) and 11.1% (4/36, range, 2 to 6) (P < 0.001), respectively. No CTC counts ≥6 in 7.5 mL of blood were detected in FIGO I patients. Additionally, a CTC count ≥6 was also strongly associated with the site (P < 0.001) and number of metastases (P < 0.001). Interestingly, compared with patients with other metastasis sites, patients with liver and brain metastases had higher CTC counts (Mann–Whitney, P < 0.001). There was a weak correlation between a CTC count ≥6 and pretreatment β-HCG level (P = 0.037), previous failed chemotherapy (P = 0.039) and surgery status (P = 0.019). The presence of ≥6 CTCs did not correlate with age (P = 0.058), antecedent pregnancy (P = 0.840), interval months from index pregnancy (P = 0.478) or largest tumor mass (P = 0.281) (ESI Table S1†). A maintained CTC count was not associated with pretreatment β-HCG level (r = −0.004; P = 0.954) or largest tumor mass (r = 0.087; P = 0.246) (ESI Fig. S2G and H†).| Patients with CTCs (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | ≥1 | ≥3 | ≥4 | ≥5 | ≥6 | ≥10 |
| Abbreviations: CTC, circulating tumor cell; P: chi-square test or Fisher's exact test. | ||||||
| All (n = 180) | 98.3 | 81.7 | 48.3 | 35.0 | 22.8 | 8.3 |
| Age (years) | ||||||
| <40 | 97.9 | 81.0 | 48.6 | 33.1 | 19.7 | 7.0 |
| ≥40 | 100.0 | 84.2 | 47.4 | 42.1 | 34.2 | 13.2 |
| P | 1.000 | 0.648 | 0.893 | 0.340 | 0.058 | 0.378 |
| FIGO score | ||||||
| ≤6 | 97.3 | 76.0 | 34.7 | 17.3 | 8.0 | 0.0 |
| >6 | 99.0 | 85.7 | 58.1 | 47.6 | 33.3 | 14.3 |
| P | 0.768 | 0.097 | 0.002 | <0.001 | <0.001 | <0.001 |
| Antecedent pregnancy | ||||||
| Mole | 99.1 | 83.2 | 47.7 | 36.4 | 24.3 | 9.3 |
| Abortion | 97.7 | 88.6 | 56.8 | 36.4 | 20.5 | 9.1 |
| Term and ectopic pregnancy | 96.6 | 65.5 | 37.9 | 27.6 | 20.7 | 3.4 |
| P | 0.359 | 0.036 | 0.280 | 0.659 | 0.840 | 0.694 |
| Interval months from index pregnancy | ||||||
| <4 | 100.0 | 80.9 | 42.6 | 31.9 | 14.9 | 6.4 |
| 4–6 | 96.3 | 77.8 | 48.1 | 33.3 | 22.2 | 3.7 |
| 7–12 | 100.0 | 87.9 | 51.5 | 33.3 | 27.3 | 12.1 |
| >12 | 97.3 | 80.8 | 50.7 | 38.4 | 26.0 | 9.6 |
| P | 0.482 | 0.758 | 0.819 | 0.889 | 0.478 | 0.707 |
| Pre-treatment β-HCG level (IU L −1 ) | ||||||
| <103 | 96.2 | 77.4 | 32.1 | 18.9 | 9.4 | 1.9 |
| 103–104 | 100.0 | 88.7 | 52.8 | 39.6 | 28.3 | 9.4 |
| 104–105 | 97.9 | 75.0 | 45.8 | 37.5 | 25.0 | 12.5 |
| >105 | 100.0 | 88.5 | 76.9 | 53.8 | 34.6 | 11.5 |
| P | 0.630 | 0.201 | 0.002 | 0.013 | 0.037 | 0.153 |
| Largest tumor mass (cm) | ||||||
| <3 | 97.4 | 77.6 | 40.8 | 26.3 | 17.1 | 5.3 |
| 3–5 | 98.8 | 84.5 | 53.6 | 41.7 | 26.2 | 11.9 |
| >5 | 100.0 | 85.0 | 55.0 | 40.0 | 30.0 | 5.0 |
| P | 0.723 | 0.488 | 0.222 | 0.112 | 0.281 | 0.268 |
| Site of metastases | ||||||
| Lungs | 97.9 | 77.4 | 37.7 | 22.6 | 8.8 | 2.1 |
| Spleen, kidneys | 100.0 | 100.0 | 100.0 | 80.0 | 60.0 | 20.0 |
| Gastrointestinal tract | 100.0 | 100.0 | 85.7 | 71.4 | 71.4 | 28.6 |
| Liver, brain | 100.0 | 100.0 | 95.5 | 95.5 | 86.4 | 40.9 |
| P | 1.000 | 0.016 | <0.001 | <0.001 | <0.001 | <0.001 |
| Number of metastases | ||||||
| 0 | 94.0 | 58.0 | 16.0 | 6.0 | 0.0 | 0.0 |
| 1–4 | 100.0 | 90.1 | 53.1 | 35.8 | 18.5 | 2.5 |
| 5–8 | 100.0 | 89.2 | 64.9 | 54.1 | 40.5 | 18.9 |
| >8 | 100.0 | 100.0 | 100.0 | 91.7 | 91.7 | 50.0 |
| P | 0.064 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Previous failed chemotherapy | ||||||
| No | 97.9 | 82.3 | 44.8 | 27.1 | 15.6 | 5.2 |
| Monotherapy | 100.0 | 72.5 | 45.0 | 35.0 | 27.5 | 7.5 |
| Combined therapy | 97.7 | 88.6 | 59.1 | 52.3 | 34.1 | 15.9 |
| P | 1.000 | 0.157 | 0.259 | 0.015 | 0.039 | 0.116 |
| Surgery | ||||||
| No | 98.0 | 81.8 | 46.5 | 30.3 | 16.2 | 5.1 |
| Yes | 98.8 | 81.5 | 50.6 | 40.7 | 30.9 | 12.3 |
| P | 1.000 | 0.954 | 0.579 | 0.144 | 0.019 | 0.078 |
| FIGO | ||||||
| I | 95.9 | 59.2 | 16.3 | 6.1 | 0.0 | 0.0 |
| II | 100.0 | 83.3 | 38.9 | 25.0 | 11.1 | 0.0 |
| III | 98.3 | 88.3 | 51.7 | 31.7 | 11.7 | 1.7 |
| IV | 100.0 | 100.0 | 97.1 | 91.4 | 85.7 | 40.0 |
| P | 0.612 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Pretreatment CTC count and survival
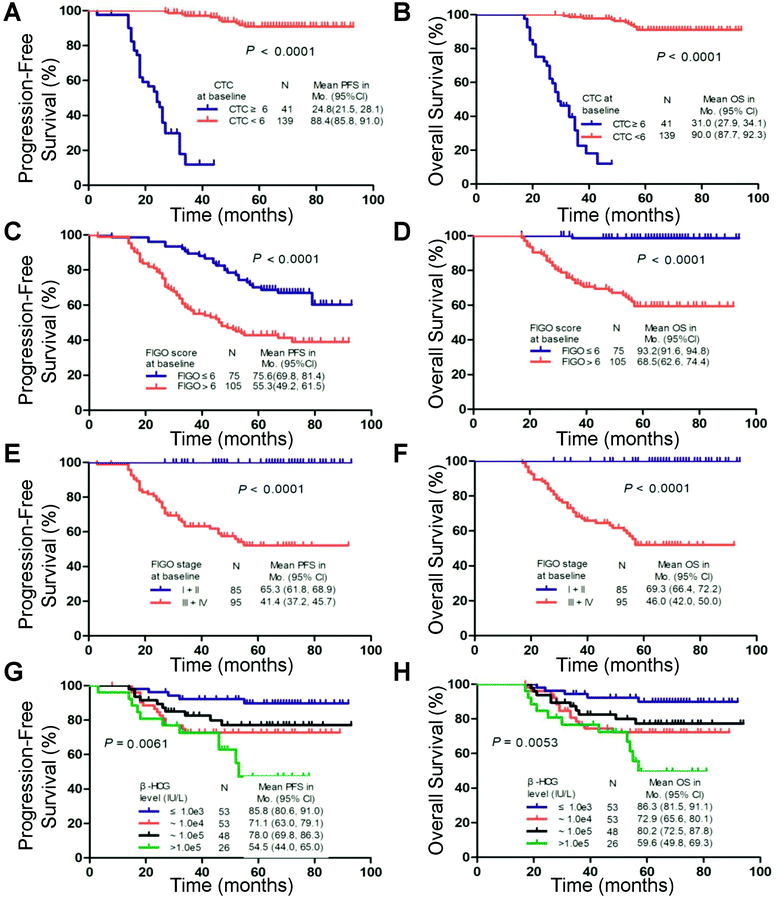
Univariate analyses revealed that clinical factors significantly associated with a poor prognosis were CTC count, age, FIGO score, interval months from index pregnancy, pretreatment β-HCG level, largest tumor mass, site of metastases, previous failed chemotherapy and FIGO stage (Table 3). As shown in the Kaplan–Meier survival curves, the presence of 6 CTCs before chemotherapy was significantly associated with PFS (median, 24.8 vs. 88.4 months; log-rank test, P < 0.001; Fig. 3) and OS (median, 31.0 vs. 90.0 months; log-rank test, P < 0.001; Fig. 3) in all patients. A multivariate analysis showed that CTC count (≥6 CTCs), FIGO score and FIGO stage were independent prognostic factors consistent with the univariate analysis (Table 3).| No. of patients | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| Risk factor | HR | 95% CI | P | HR | 95% CI | P | |
| Abbreviation: CTC, circulating tumor cell.a Overall P value. For multivariate analyses, a stepwise method was used to select the variables with statistical significance. | |||||||
| Univariate analyses | |||||||
| CTC count | |||||||
| <6 | 139 | 1.0 | 1.0 | ||||
| ≥6 | 41 | 65.0 | 23.3–181.3 | <0.001 | 62.6 | 21.2–184.8 | <0.001 |
| Age (years) | |||||||
| <40 | 142 | 1.0 | 1.0 | ||||
| ≥40 | 38 | 2.5 | 1.3–4.8 | 0.005 | 2.5 | 1.3–4.7 | 0.006 |
| FIGO score | |||||||
| ≤6 | 75 | 1.0 | 1.0 | ||||
| >6 | 105 | 35.7 | 4.9–260.1 | <0.001 | 35.6 | 4.9–259.2 | <0.001 |
| FIGO stage | |||||||
| I + II | 85 | 1.0 | 1.0 | ||||
| III + IV | 95 | 84.9 | 6.5–1114.9 | 0.001 | 88.7 | 6.7–1176.6 | 0.001 |
| Antecedent pregnancy | |||||||
| Mole | 107 | 1.0 | 1.0 | ||||
| Abortion | 44 | 0.8 | 0.4–1.8 | 0.8 | 0.4–1.8 | ||
| Term and ectopic pregnancy | 29 | 1.1 | 0.5–2.5 | 0.777a | 1.1 | 0.5–2.5 | 0.791a |
| Interval months from index pregnancy | |||||||
| <4 | 47 | 1.0 | 1.0 | ||||
| 4–6 | 27 | 0.9 | 0.2–4.9 | 0.9 | 0.2–4.9 | ||
| 7–12 | 33 | 2.6 | 0.8–9.0 | 2.7 | 0.8–9.2 | ||
| >12 | 73 | 4.9 | 1.7–14.0 | 0.004a | 4.9 | 1.7–13.9 | 0.004a |
| Pre-treatment β-HCG level (IU L −1 ) | |||||||
| <103 | 53 | 1.0 | 1.0 | ||||
| 103–104 | 53 | 3.3 | 1.2–9.0 | 3.3 | 1.2–9.1 | ||
| 104–105 | 48 | 2.5 | 0.8–7.2 | 2.5 | 0.9–7.3 | ||
| >105 | 26 | 5.7 | 2.0–16.3 | 0.013a | 5.6 | 2.0–16.3 | 0.013a |
| Largest tumor mass (cm) | |||||||
| <3 | 76 | 1.0 | 1.0 | ||||
| 3–5 | 84 | 3.1 | 1.4–6.9 | 3.2 | 1.4–7.0 | ||
| >5 | 20 | 3.7 | 1.3–10.1 | 0.012a | 3.7 | 1.3–10.3 | 0.010a |
| Site of metastases | |||||||
| Lungs | 91 | 1.0 | 1.0 | ||||
| Spleen, kidneys | 5 | 3.0 | 0.4–22.4 | 3.5 | 0.5–26.9 | ||
| Gastrointestinal tract | 7 | 8.7 | 1.9–39.2 | 6.6 | 1.5–29.7 | ||
| Liver, brain | 23 | 23.3 | 11.4–47.7 | <0.001a | 27.3 | 13.2–56.6 | <0.001a |
| Number of metastases | |||||||
| 0 | 49 | 1.0 | 1.0 | ||||
| ≥1 | 131 | 39.4 | 2.5–622.0 | 0.009 | 39.9 | 2.5–625.2 | 0.009 |
| Previous failed chemotherapy | |||||||
| No | 96 | 1.0 | 1.0 | ||||
| Monotherapy | 40 | 2.8 | 1.1–7.3 | 2.8 | 1.1–7.3 | ||
| Combined therapy | 44 | 7.5 | 3.3–16.7 | <0.001a | 7.3 | 3.3–16.4 | <0.001a |
| Surgery | |||||||
| No | 99 | 1.0 | 1.0 | ||||
| Yes | 81 | 1.4 | 0.8–2.7 | 0.246 | 1.4 | 0.8–2.6 | 0.272 |
| Multivariate analyses | |||||||
| CTC count | |||||||
| <6 | 139 | 1.0 | 1.0 | ||||
| ≥6 | 41 | 14.9 | 4.3–51.2 | <0.001 | 15.8 | 4.3–57.9 | <0.001 |
| FIGO score | |||||||
| ≤6 | 75 | 1.0 | 1.0 | ||||
| >6 | 105 | 11.8 | 1.5–90.8 | <0.001 | 11.1 | 1.5–84.6 | 0.020 |
| FIGO stage | |||||||
| I + II | 85 | 1.0 | 1.0 | ||||
| III + IV | 95 | 3.9 | 1.9–8.0 | <0.001 | 5.5 | 2.6–11.9 | <0.001 |
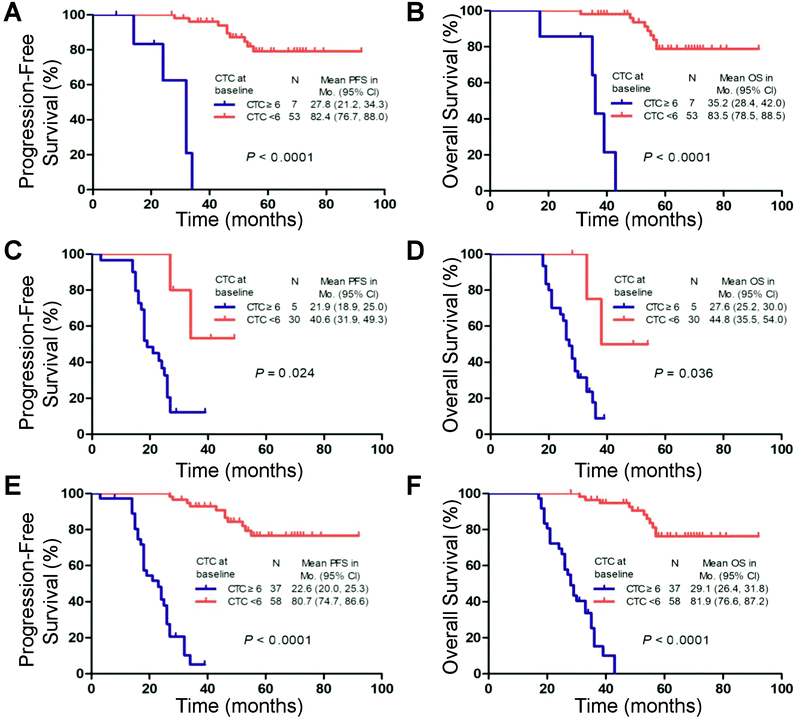
The Kaplan–Meier analysis demonstrated that ≥6 CTCs predicted decreased PFS and OS compared with patients with <6 CTCs in the FIGO III (P < 0.001 and P < 0.001), FIGO IV (P = 0.024 and P = 0.016) and FIGO III and IV subgroups (P < 0.001 and P < 0.001), respectively (Fig. 4). These findings were confirmed by the univariate analysis (ESI Table S4†).
Post-treatment CTC count and survival
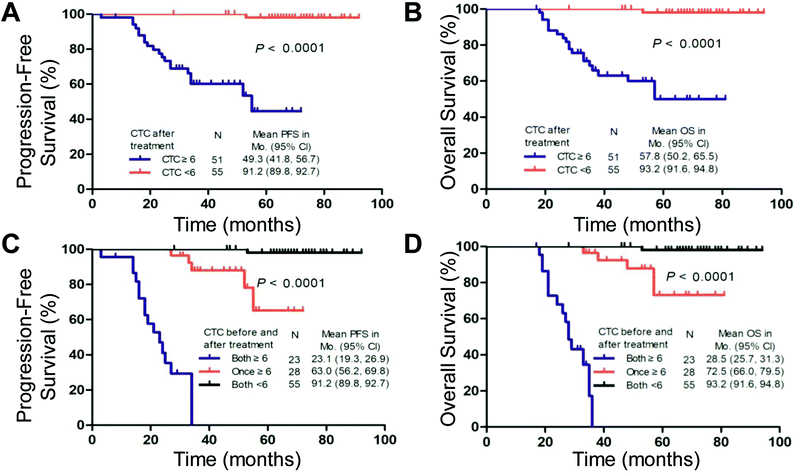
The PFS and OS of the patients with ≥6 CTCs at the second time point were significantly shorter than those of the patients with <6 CTCs (median, 49.3 vs. 91.2 months and 57.8 vs. 93.2 months, respectively; log-rank test P < 0.001; Fig. 5A and B). Regarding consideration of the pre- and post-treatment CTC counts together, ≥6 CTCs and <6 CTCs at both time points (before and after one cycle of chemotherapy) were observed in 23 cases and 55 cases, respectively. In the other 28 cases, ≥6 CTCs were measured at one of the time points. Compared with patients with ≥6 CTCs at either time point, patients with <6 CTCs at both time points had higher PFS (91.2 vs. 63.0 months; log-rank test P < 0.001) and OS (93.2 vs. 72.5 months; log-rank test P < 0.001). Patients with ≥6 CTCs at both time points had the worst prognosis (median PFS, 23.1 months; median OS, 28.5 months), consistent with the univariate analysis (Fig. 5C and D and ESI Table S5†).Discussion
The association of the CTC count with poor outcome has been widely demonstrated in metastatic breast, colorectal, prostate and gastric cancers.10,17,18 In the present study, we provided for the first time a proof of concept of the prognostic significance of the CTC count in a large population of GC patients. Approximately 96% of GC patients contained ≥2 CTCs in 7.5 mL of blood, which is significantly higher than the rates observed for other tumor types.19 In addition, 100% of FIGO IV patients had ≥2 CTCs detected before the chemotherapy treatment. The high detection rate may be attributed to the hematogenous dissemination behavior and the high combined expression of EpCAM and CD147 in GC.13,14The HRs and differences in 3-year PFS and OS were high for five or seven cells in our training set, but they reached a maximum for a threshold of six. Thus, it may be justified to define an appropriate threshold for the unfavorable GC subgroup of ≥6 CTCs per 7.5 mL, which is much higher than the ≥3 CTCs per 7.5 mL used for colon cancer and ≥5 CTCs per 7.5 mL used for metastatic breast and prostate cancers.10,18–20 The number of epithelial cells in the peripheral blood of healthy volunteers and patients with benign disease is extremely low and almost never exceeds 1 cell per 7.5 mL of blood.19 Hence, a high cutoff value of 6 CTCs will statistically decrease the risk of assigning patients to the wrong prognostic group when stratifying patients with different prognoses.
The pretreatment presence of 6 CTCs was significantly correlated with the FIGO score, metastasis site, metastasis number, and FIGO stage. The multivariate Cox regression analysis also revealed that the pretreatment CTC count was an independent risk factor for PFS and OS, with a 14.9-fold increased risk of progression and a 15.8-fold increased risk of death in those patients with six CTCs at the baseline. Moreover, along with GC progression, as reflected by the FIGO stage, the percentage of patients with ≥6 CTCs increased gradually. For FIGO stage III or IV patients, the presence of 6 CTCs before treatment could effectively differentiate PFS and OS in the univariate analysis. Classical anatomical prognostic factors were included in the revised FIGO 2000 Classification of Gestational Trophoblastic Neoplasia.21 Therefore, the CTC count, as an indirect indicator of the anatomical metastasis status,22 may assist patient stratification for FIGO staging at the time of GC diagnosis. Our results support the role of the CTC count in assessing the metastasis status in GC and suggest that patients with CTC counts ≥6 have an increased risk of distant multiple-organ metastases. Although metastasis to the lungs is the most common in GC, patients with cerebral metastases often present with severe neurological symptoms as a result of intracranial bleeding or increased intracranial pressure.23 Making a preoperative diagnosis using a single tissue biopsy is very difficult if the metastasis site is located in the mediastinum, pineal gland or retroperitoneum.24,25 Thus, as a representative of the primary tumor location and various metastatic sites,26 “liquid CTC biopsy” can not only reflect the metastatic process of GC but also provide more information regarding biomarker status than a single tissue biopsy taken at a given time.
Clinically, approximately 30% of patients, considered at a low risk of acquiring drug resistance based on having a FIGO score of 6 or less, eventually develop resistance to single-agent chemotherapy with methotrexate or dactinomycin.27 The chemotherapy regimen for GC is based on the FIGO prognostic score, which may not dynamically and truly represent a heterogeneous tumor. Traditionally, β-HCG has served as an ideal tumor marker for GC diagnosis and disease status evaluation.5 However, a growing body of evidence concerning false-positive test results raises concerns for the future clinical application of β-HCG, creating the demand for a new indicator for GC patients.28 Our data support the FIGO score and β-HCG level as prognostic markers, but compared with a pretreatment CTC count of 6 as a dichotomous variable, these markers exhibit poor discrimination in univariate and multivariate analyses.
Currently, there are no internationally recognized guidelines regarding when to stop chemotherapy for GC after biochemical remission. Some experts recommend stopping chemotherapy immediately when serum β-HCG becomes undetectable, especially for low-risk GC patients. Others suggest providing an additional two courses past the first normal serum β-HCG result.29 Our study demonstrated that GC patients with <6 CTCs at both time points had longer PFS than those with ≥6 CTCs at either time point. Thus, a simple CTC count assessment could be used to evaluate whether patients are benefiting from a current chemotherapy regimen. If the CTC count in GC patients remains or becomes ≥6 after one cycle of chemotherapy, an alternative regimen may be essential.
CTCs, as a new biomarker, can also further the understanding of the key biological mechanisms underlying their growth and dissemination.30 However, their applications in the early diagnosis, evaluation and management of preoperative systemic therapies, as well as the post-surgical dynamic detection of minimal residual disease and cancer relapse, require intensive clinical exploration. Next, we will focus primarily on GC to illustrate many of the above issues through a prospective clinical trial, largely because the hematogenous dissemination of GC guarantees enough CTC samples for researching various time points. Recently, several studies have reported short-term (≤28 days) or long-term (6–24 months) in vitro cultures of CTCs from patients with advanced cancer,31–33 and these model systems open exciting possibilities for the use of CTC genotyping and function testing to evaluate the efficacy of different drug combinations in GC patients.
Conclusions
In conclusion, this is the first study on the prognostic significance of CTCs in GC patients. CTC detection as a liquid biopsy could be useful for assisting the stratification of high-risk GC patients for early intervention and dynamic treatment efficacy evaluation.Author contributions
Conception and design: Zunfu Ke, Chunlin Wang, Hui Zhang, Zhiming Ding, and Chao Zeng.Provision of study materials or patients: Chunlin Wang, Jessica Cao, Yi Zhang, Yang Zhang, Minzhi Hou, Shanyang He, Hongwei Shen, Jing Liu, Manman Xu and Shijun Sun.
Data analysis and interpretation: Xinlin Chen, Yongjiang Zheng, Cong Sun, Wenting Jiang, Han Wang, Neng Jiang, Yongmei Cui, Yu Sun, Yangshan Chen, Mengzhen Li, Jianhong Wang and Millicent Lin.
Manuscript writing: Zunfu Ke and Jessica Cao.
Final approval of manuscript: All authors.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from 2017YFC1308800, the National Natural Science Foundation of China (30900650, 81372501, 81572260, 81773299 and 31430030), the Guangdong Natural Science Foundation (2011B031800025, S2012010008378 and 2015A030313036), and the Guangzhou and Guangdong/Guangzhou Science and Technology Planning Program (2014J4100132, 2012B031800115, 2013B02180021, 2015A020214010, 2016A020215055, 2016A020215127, 201704020094, 16ykjc08 and 2015ykzd07).References
- P. Savage, M. Seckl and D. Short, J. Reprod. Med., 2008, 53, 774–780 Search PubMed.
- C. Alifrangis, R. Agarwal, D. Short, R. A. Fisher, N. J. Sebire, R. Harvey, P. M. Savage and M. J. Seckl, J. Clin. Oncol., 2013, 31, 280–286 CrossRef CAS PubMed.
- M. S. Cagayan and L. R. Lu-Lasala, J. Reprod. Med., 2006, 51, 785–792 Search PubMed.
- I.-M. Shih, Lancet Oncol., 2007, 8, 642–650 CrossRef CAS PubMed.
- K. G. Essel, A. Bruegl, D. M. Gershenson, L. M. Ramondetta, R. W. Naumann and J. Brown, Gynecol. Oncol., 2017, 146, 74–80 CrossRef CAS PubMed.
- T. Masuda, N. Hayashi, T. Iguchi, S. Ito, H. Eguchi and K. Mimori, Mol. Oncol., 2016, 10, 408–417 CrossRef CAS PubMed.
- F.-C. Bidard, D. J. Peeters, T. Fehm, F. Nolé, R. Gisbert-Criado, D. Mavroudis, S. Grisanti, D. Generali, J. A. Garcia-Saenz, J. Stebbing, C. Caldas, P. Gazzaniga, L. Manso, R. Zamarchi, A. F. de Lascoiti, L. De Mattos-Arruda, M. Ignatiadis, R. Lebofsky, S. J. van Laere, F. Meier-Stiegen, M.-T. Sandri, J. Vidal-Martinez, E. Politaki, F. Consoli, A. Bottini, E. Diaz-Rubio, J. Krell, S.-J. Dawson, C. Raimondi, A. Rutten, W. Janni, E. Munzone, V. Carañana, S. Agelaki, C. Almici, L. Dirix, E.-F. Solomayer, L. Zorzino, H. Johannes, J. S. Reis-Filho, K. Pantel, J.-Y. Pierga and S. Michiels, Lancet Oncol., 2014, 15, 406–414 CrossRef PubMed.
- M. G. Krebs, R. L. Metcalf, L. Carter, G. Brady, F. H. Blackhall and C. Dive, Nat. Rev. Clin. Oncol., 2014, 11, 129 CrossRef CAS PubMed.
- J. B. Benedet, Int. J. Gynaecol. Obstet., 2006, 95(Suppl 1), S1–257 CrossRef PubMed.
- S. J. Cohen, C. J. A. Punt, N. Iannotti, B. H. Saidman, K. D. Sabbath, N. Y. Gabrail, J. Picus, M. Morse, E. Mitchell, M. C. Miller, G. V. Doyle, H. Tissing, L. W. M. M. Terstappen and N. J. Meropol, J. Clin. Oncol., 2008, 26, 3213–3221 CrossRef PubMed.
- S. Wang, H. Wang, J. Jiao, K.-J. Chen, G. E. Owens, K.-i. Kamei, J. Sun, D. J. Sherman, C. P. Behrenbruch, H. Wu and H.-R. Tseng, Angew. Chem., Int. Ed., 2009, 48, 8970–8973 CrossRef CAS PubMed.
- Y.-S. Hsiao, S.-C. Luo, S. Hou, B. Zhu, J. Sekine, C.-W. Kuo, D.-Y. Chueh, H. Yu, H.-R. Tseng and P. Chen, Small, 2014, 10, 3012–3017 CrossRef CAS PubMed.
- S. Schonberger, V. Okpanyi, G. Calaminus, S. Heikaus, I. Leuschner, J. C. Nicholson, N. H. Stoecklein, D. T. Schneider and A. Borkhardt, Genes, Chromosomes Cancer, 2013, 52, 24–32 CrossRef PubMed.
- M. Singh, D. Kindelberger, Z. Nagymanyoki, S. W. Ng, C. M. Quick, H. Yamamoto, R. Fichorova, V. Fulop and R. S. Berkowitz, J. Reprod. Med., 2012, 57, 197–203 CAS.
- W. Chen, S. Weng, F. Zhang, S. Allen, X. Li, L. Bao, R. H. W. Lam, J. A. Macoska, S. D. Merajver and J. Fu, ACS Nano, 2013, 7, 566–575 CrossRef CAS PubMed.
- S. Liu, Z. Tian, L. Zhang, S. Hou, S. Hu, J. Wu, Y. Jing, H. Sun, F. Yu, L. Zhao, R. Wang, H.-R. Tseng, H. E. Zhau, L. W. K. Chung, K. Wu, H. Wang, J. B. Wu, Y. Nie and C. Shao, Oncotarget, 2016, 7, 59877–59891 Search PubMed.
- S. Matsusaka, K. Chin, M. Ogura, M. Suenaga, E. Shinozaki, Y. Mishima, Y. Terui, N. Mizunuma and K. Hatake, Cancer Sci., 2010, 101, 1067–1071 CrossRef CAS PubMed.
- W. J. Allard, L. W. M. M. Terstappen and D. F. Hayes, N. Engl. J. Med., 2004, 351, 781–791 CrossRef.
- W. J. Allard, J. Matera, M. C. Miller, M. Repollet, M. C. Connelly, C. Rao, A. G. J. Tibbe, J. W. Uhr and L. W. M. M. Terstappen, Clin. Cancer Res., 2004, 10, 6897 CrossRef PubMed.
- J. S. de Bono, H. I. Scher, R. B. Montgomery, C. Parker, M. C. Miller, H. Tissing, G. V. Doyle, L. W. W. M. Terstappen, K. J. Pienta and D. Raghavan, Clin. Cancer Res., 2008, 14, 6302 CrossRef CAS PubMed.
- H. Y. S. Ngan, Int. J. Gynaecol. Obstet., 2002, 77, 285–287 Search PubMed.
- M. Ignatiadis, M. Lee and S. S. Jeffrey, Clin. Cancer Res., 2015, 21, 4786 CrossRef CAS PubMed.
- W. Small, J. R. Lurain, R. M. Shetty, C. F. Huang, G. L. Applegate and W. N. Brand, Radiology, 1996, 200, 277–280 CrossRef PubMed.
- K. Minamino, Y. Adachi, A. Okamura, T. Kushida, M. Sugi, M. Watanabe, K. Muguruma, H. Sugao, Y. Suzuki, M. Iwasaki, K. Nakano, Y. Koike, J. Wang, H. Mukaide, Y. Zhang, T. Matsuda, M. Matsumura and S. Ikehara, Pathol. Int., 2005, 55, 216–222 CrossRef.
- F. E. Franke, K. Pauls, L. Kerkman, K. Steger, T. Klonisch, R. Metzger, F. Alhenc-Gelas, E. Burkhardt, M. Bergmann and S. M. Danilov, Hum. Pathol., 2000, 31, 1466–1476 CrossRef CAS PubMed.
- E. Pailler, J. Adam, A. Barthélémy, M. Oulhen, N. Auger, A. Valent, I. Borget, D. Planchard, M. Taylor, F. André, J. C. Soria, P. Vielh, B. Besse and F. Farace, J. Clin. Oncol., 2013, 31, 2273–2281 CrossRef PubMed.
- I. A. McNeish, S. Strickland, L. Holden, G. J. Rustin, M. Foskett, M. J. Seckl and E. S. Newlands, J. Clin. Oncol., 2002, 20, 1838–1844 CrossRef CAS PubMed.
- T. Y. Ng and L. C. Wong, Best Pract. Res. Clin. Obstet. Gynaecol., 2003, 17, 893–903 CrossRef CAS.
- R. Nadhan, J. V. Vaman, C. Nirmala, S. Kumar Sengodan, S. Krishnakumar Hemalatha, A. Rajan, G. R. Varghese, N. Rl, A. K. Bv, R. Thankappan and P. Srinivas, Crit. Rev. Oncol. Hematol., 2017, 114, 77–90 CrossRef PubMed.
- L. Cabel, C. Proudhon, P. Mariani, D. Tzanis, G. Beinse, I. Bieche, J. Y. Pierga and F. C. Bidard, Eur. J. Surg. Oncol., 2017, 43, 949–962 CrossRef CAS PubMed.
- M. Yu, A. Bardia, N. Aceto, F. Bersani, M. W. Madden, M. C. Donaldson, R. Desai, H. Zhu, V. Comaills, Z. Zheng, B. S. Wittner, P. Stojanov, E. Brachtel, D. Sgroi, R. Kapur, T. Shioda, D. T. Ting, S. Ramaswamy, G. Getz, A. J. Iafrate, C. Benes, M. Toner, S. Maheswaran and D. A. Haber, Science, 2014, 345, 216–220 CrossRef CAS.
- D. Gao, I. Vela, A. Sboner, P. J. Iaquinta, W. R. Karthaus, A. Gopalan, C. Dowling, J. N. Wajala, E. A. Undvall, V. K. Arora, J. Wongvipat, M. Kossai, S. Ramazanoglu, L. P. Barboza, W. Di, Z. Cao, Q. F. Zhang, I. Sirota, L. Ran, T. Y. MacDonald, H. Beltran, J.-M. Mosquera, K. A. Touijer, P. T. Scardino, V. P. Laudone, K. R. Curtis, D. E. Rathkopf, M. J. Morris, D. C. Danila, S. F. Slovin, S. B. Solomon, J. A. Eastham, P. Chi, B. Carver, M. A. Rubin, H. I. Scher, H. Clevers, C. L. Sawyers and Y. Chen, Cell, 2014, 159, 176–187 CrossRef CAS.
- L. Cayrefourcq, T. Mazard, S. Joosse, J. Solassol, J. Ramos, E. Assenat, U. Schumacher, V. Costes, T. Maudelonde, K. Pantel and C. Alix-Panabières, Cancer Res., 2015, 75, 892 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8bm01130c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |