The influence of electrically conductive and non-conductive nanocomposite scaffolds on the maturation and excitability of engineered cardiac tissues†
Abstract
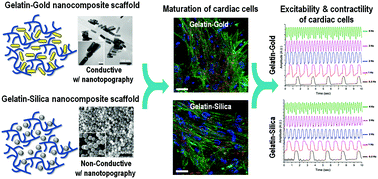
Utilization of electrically conductive nanomaterials for developing nanocomposite scaffolds has been at the center of attention for engineering functional cardiac tissues. The primary motive in the use of conductive nanomaterials has been to develop biomimetic scaffolds to recapitulate the extracellular matrix (ECM) of the native heart and to promote cardiac tissue maturity, excitability and electrical signal propagation. Alternatively, it is well accepted that the inclusion of nanomaterials also alters the stiffness and nano-scale topography of the scaffolds. However, what is missing in the literature is that to what extent the sole presence of nanomaterials within a scaffold, regardless of their conductivity, influences the maturation and excitability of engineered cardiac tissues. To address this knowledge gap, we developed four different classes of gelatin methacrylate (GelMA) hydrogels, with varied concentrations, embedded electrically conductive gold nanorods (GNRs) and non-conductive silica nanomaterials (SNPs), to assess the influence of matrix stiffness and the presence of nanomaterials on cardiac cell adhesion, protein expression (i.e. maturation), and tissue-level excitability. Our results demonstrated that either embedding nanomaterials (i.e. GNRs and SNPs) or increasing the matrix stiffness significantly promoted cellular retention and the expression of cardiac-specific markers, including sarcomeric α-actinin (SAC), cardiac troponin I (cTnI) and connexin43 (Cx43) gap junctions. Notably, excitation voltage thresholds at a high frequency (i.e. 2 Hz and higher), in both coupled and uncoupled gap junctions induced by heptanol, were lower for scaffolds embedded conductive GNRs or non-conductive SNPs, independent of matrix stiffness. Overall, our findings demonstrated that the sole presence of nanomaterials within the scaffolding matrix had a more pronounced influence as compared to the scaffold stiffness on the cell–cell coupling, maturation and excitability of engineered cardiac tissues.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...