Spontaneous implantation of gold nanoparticles on graphene oxide for salivary SERS sensing†
Abstract
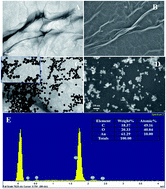
Clean SERS substrates could be prepared without using polymer stabilizers and highly toxic reducing agents, and they play a pivotal role in trace amount detection. Herein, well-dispersed graphene oxide (GO)-supported Au (GO–Au) nanocomposites are synthesized using a hydrothermal method in an alkaline environment in the absence of traditionally used reductants and surfactants. These nanocomposites are subsequently used to manufacture a SERS sensor. The silk-like GO plays multiple roles as the reductant for the synthesis of Au nanoparticles (NPs), the surfactant for Au NPs and the support to uniformly distribute Au NPs. Moreover, the in situ obtained Au NPs could provide efficient ‘hot spots’, and therefore ensure favorable SERS analytical performance of the GO–Au nanocomposite-based sensor which featured an enhancement factor of 8.1 × 107. To further evaluate its practical clinical applications, the prepared SERS sensor was utilized to analyze eight salivary amino acids, which are gastric cancer (GC) biomarkers, in 44 saliva samples. The results revealed that the performance of the prepared sensor was satisfactory for salivary SERS sensing and the selectivity and sensitivity of the sensor for diagnosing GC were excellent. Therefore, this simple synthesis strategy could provide a novel idea for preparing clean SERS sensor substrates with potential application prospects in the clinical field.


 Please wait while we load your content...
Please wait while we load your content...