Development of highly selective potentiometric thorium(iv) ion-selective electrode: exploration supported with optical and DFT analysis†
Abstract
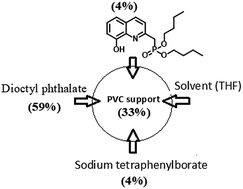
A new liquid membrane based on poly(vinyl chloride) composed of dibutyl(8-hydroxyquinolin-2-yl)methylphosphonate (L) as a neutral ion carrier, sodium tetraphenylborate (NaTPB) as a lipophilic salt dissolved in dioctyl phthalate (DOP) as a plasticizer-cum-membrane solvent was developed for the selective determination of Th4+. The best electrode response for Th4+ is achieved with the membrane composition of PVC : DOP : L : NaTPhB (33% : 59% : 4% : 4%, w/w). The electrode response follows a super-Nernstian response with a slope 31.2 ± 0.4 mV per decade at 25 °C over a wide concentration range of Th4+ from 1 × 10−7 to 1 × 10−1 M. The electrode has a response time of 5 s and detection limit of 1 × 10−8 M, working in the pH range of 4 to 6.5, with a service lifetime of about three months. The selectivity coefficient was determined by the match potential method (MPM) and the results show good selectivity towards Th4+ over a wide variety of selected metal ions (from s, d and f-block metals). The binding mechanism was investigated by FTIR, NMR (1H, 31P) and mass analysis complemented by density functional theoretical results.


 Please wait while we load your content...
Please wait while we load your content...