Visual detection of Zika virus by isothermal nucleic acid amplification combined with a lateral-flow device
Abstract
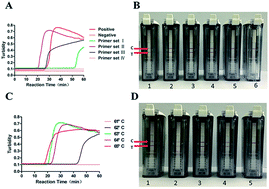
The Zika virus (ZIKV) did not receive significant attention in the past until the ZIKV outbreak occurred a few years ago. It has been shown that ZIKV can trigger congenital microcephaly, Guillain–Barré syndrome and other neurological syndromes. To fight against ZIKV, the efficient diagnosis of ZIKV is absolutely required; this has prompted us to establish a visual detection method for ZIKV with high accuracy and sensitivity. We applied reverse transcription loop-mediated isothermal amplification (RT-LAMP) with primers targeted to the specific conserved region of the non-structural protein 5 (NS5) gene fragment; moreover, using a lateral flow device (LFD), the detection of the ZIKA genome was completed within 1 hour in a 65 °C water bath. Compared with one-step real-time PCR (one-step RT-PCR), a RT-LAMP-turbidimeter, and quantitative reverse transcription PCR (RT-qPCR), our method is more convenient, sensitive, and specific, less time-consuming, and has equal detection performance. The newly developed method was evaluated for 12 clinical serum samples, and the results were consistent with the previous RT-qPCR detection results obtained by the Centers for Disease Control and Prevention of Guangdong; this supported that the developed method could be a potential solution for ZIKV diagnosis.
- This article is part of the themed collection: Coronavirus articles - free to access collection


 Please wait while we load your content...
Please wait while we load your content...