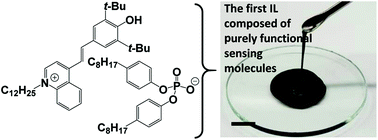
An ionic liquid composed of purely functional sensing molecules: a colorimetrically calcium responsive ionic liquid †
Abstract
A highly lipophilic ionic liquid (IL) consisting of stoichiometrically equal amounts of two purely functional chemical sensing molecules, an anionic calcium ionophore and a cationic dye, was synthesized for the first time, and used as a component for a poly(vinyl chloride)-based or neat liquid membrane optical sensor. This is the first report of an IL consisting of purely functional chemical sensing molecules, which is completely different from the previously reported ionic liquids typically consisting of imidazolium or other lipophilic cations. Since the present IL contained an extremely high concentration of dyes, the IL-based sensor showed dramatically enhanced sensitivity (13 times higher compared to that of a conventional sensor), and fully reversible and selective response to Ca2+. Preliminary investigation on the unique response characteristics of the present liquid membrane IL-based sensor was performed with the responses to Ca2+ and Na+.

 Please wait while we load your content...
Please wait while we load your content...