A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays
Abstract
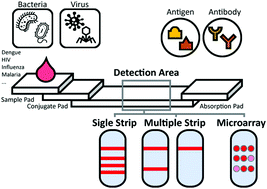
Infectious diseases are transmissible or communicable illnesses and can spread quickly in some areas and become epidemics. It is critical to quickly diagnose initial infections and prevent further spread through in vitro diagnosis. However, current detection strategies have exhibited a lack of balance with regard to accuracy, time consumption, and portability until recently (e.g. serology, culturing, molecular tests, etc.). Alternatively, many studies have focused on point-of-care testing (POCT), which combines simple, rapid, and exact on-site diagnostic platforms. Moreover, multiplex detectability is necessary for emergency treatment depending on the stage of the disease or interactional infections. The lateral flow assay (LFA) is the most popular diagnostic tool that meets the required standards for colorimetric assays. Here, we review lateral flow assays based on the immune reactions for the simultaneous diagnosis of infectious diseases as the POC test. The assays employed various forms and approaches in terms of the multiplexing level system for improving the sensitivity and specificity. We briefly describe the state-of-the-art infection diagnostic methods and published performances that have been classified into three categories based on the application forms of the lateral flow immunoassay. Also, we discuss further uses of LFA and other technologies for more effective infectious disease POCT.
- This article is part of the themed collections: Coronavirus articles - free to access collection and Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...