Single cell analysis of aged RBCs: quantitative analysis of the aged cells and byproducts†
Abstract
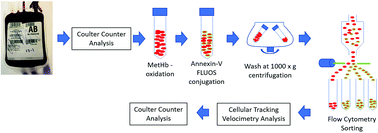
This study initially focused on characterizing the aging process of red blood cells by correlating the loss of hemoglobin and the translocation of phosphatidylserine (PS) in expired human red blood cells, hRBCs. Five pre-storage, leukoreduced hRBC units in AS-5 solution were stored between 1 and 6 °C for 42 days. Aliquots from each of these units were stained with Annexin-V FLUOS, which binds to externalized PS, and the hemoglobin within the cells was placed in a methemoglobin state with sodium nitrite, metHb. These aliquots were subsequently sorted into four sub-populations, ranging from no PS expression to high PS expression using a BD FACS ARIAIII. Each of these sub-fractions were introduced into the cell tracking velocimetry apparatus which measured both the magnetically-induced and the gravity-induced velocity. Subsequently, the samples were removed from the cell tracking velocimetry instrument and characterized using the Multisizer 4e Coulter Counter. From the magnetically-induced velocity, the amount of hemoglobin, in pg Hb per cell can be determined, and using an average value of the density of RBCs, the size can be determined. For the PS negative sub-fraction of RBCs, the size of the RBC was as expected but the average hemoglobin, Hb, content was below the threshold which defines anemia. In contrast, unexpected results were observed with the various levels of expression of PS. First, virtually all of the PS expressing cells were significantly smaller, on the order of 1 micron, than a normal RBC after 42 days of storage; yet the density of these small cells/microvesicles was such that they had settling velocities similar to normal-sized RBCs. Further, while the total amount of Hb per small cell/microvesicle was only approximately 25% of the full-sized RBCs, the volume of these small cells/microvesicles is only 1/200 of the PS negative RBCs. This suggests that these PS expressing cells are shrunken RBCs, or shrunken microvesicles from RBCs that concentrated the Hb internally. These results suggest not only a relationship between the loss of hemoglobin and the amount of PS exposed on the cellular outer wall, but also a mechanism by which these aged RBCs break down. It is not known at this time whether this is an artifact of storage or similar mechanisms occur in circulation within the human body.
- This article is part of the themed collection: Next wave advances in single cell analyses


 Please wait while we load your content...
Please wait while we load your content...