Recent advances in single-cell analysis by mass spectrometry
Abstract
Cells are the most basic structural units that play vital roles in the functioning of living organisms. Analysis of the chemical composition and content of a single cell plays a vital role in ensuring precise investigations of cellular metabolism, and is a crucial aspect of lipidomic and proteomic studies. In addition, structural knowledge provides a better understanding of cell behavior as well as the cellular and subcellular mechanisms. However, single-cell analysis can be very challenging due to the very small size of each cell as well as the large variety and extremely low concentrations of substances found in individual cells. On account of its high sensitivity and selectivity, mass spectrometry holds great promise as an effective technique for single-cell analysis. Numerous mass spectrometric techniques have been developed to elucidate the molecular profiles at the cellular level, including electrospray ionization mass spectrometry (ESI-MS), secondary ion mass spectrometry (SIMS), laser-based mass spectrometry and inductively coupled plasma mass spectrometry (ICP-MS). In this review, the recent advances in single-cell analysis by mass spectrometry are summarized. The strategies of different ionization modes to achieve single-cell analysis are classified and discussed in detail.
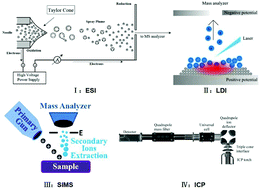
- This article is part of the themed collections: Recent Review Articles, In celebration of Chinese New Year 2020, Recent analytical chemistry science from China and Next wave advances in single cell analyses


 Please wait while we load your content...
Please wait while we load your content...