Single-cell assay on microfluidic devices
Abstract
Advances in microfluidic techniques have prompted researchers to study the inherent heterogeneity of single cells in cell populations. This would be helpful in the identification of major diseases and the design of personalized medicine. Different microfluidic approaches provide a variety of functions in the process of single-cell analysis. In this review, we take a broad overview of various microfluidic-based approaches for single-cell isolation, single-cell lysis, and single-cell analysis. Up-to-date flagship techniques and the pros and cons of these methods are discussed in detail.
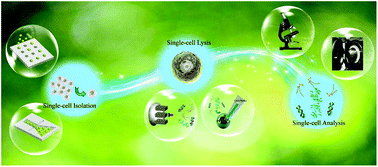
- This article is part of the themed collections: Recent Review Articles, In celebration of Chinese New Year 2020 and Next wave advances in single cell analyses


 Please wait while we load your content...
Please wait while we load your content...