Monolithic heteronanomat paper air cathodes toward origami-foldable/rechargeable Zn–air batteries†
Abstract
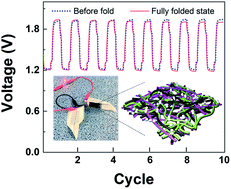
The ongoing surge in demand for flexible/wearable electronics spurs us to explore high-performance power sources with various form factors. Here we demonstrate monolithic heteronanomat (MH) paper air cathodes as a new electrode platform to enable the fabrication of origami-foldable zinc (Zn)–air batteries with reliable electrochemical rechargeability. The MH paper air cathodes consist of one-dimensional (1D) bifunctional catalyst mixtures (NdBa0.5Sr0.5Co1.5Fe0.5O5+δ double perovskite (NBSCF) nanofibers for the oxygen evolution reaction and nitrogen-doped carbon nanotubes (N-CNTs) for the oxygen reduction reaction), cellulose nanofibers (CNFs), and polytetrafluoroethylene (PTFE) nanoparticles, without the incorporation of conventional current collectors and gas diffusion layers. The CNFs and PTFE nanoparticles act as hydrophilic and hydrophobic binders, respectively, to construct three-dimensional (3D) bicontinuous electrolyte/air channels in the MH paper air cathodes. The well-developed electrolyte/air transport pathways, in combination with the rational design of the 1D bifunctional catalyst mixtures, enables the resultant Zn–air batteries (MH paper air cathode|CNF separator membrane|Zn–foil anode) to exhibit highly efficient charge/discharge performance and cyclability. In addition, the highly entangled network structure (based on a fibrous mixture of NBSCFs, N-CNTs, and CNFs) of the MH paper air cathode substantially improves its mechanical flexibility under various deformation modes, enabling the resultant Zn–air battery to be folded into a paper-airplane shape via origami folding.


 Please wait while we load your content...
Please wait while we load your content...