Biocatalytic dynamic kinetic reductive resolution with ketoreductase from Klebsiella pneumoniae: the asymmetric synthesis of functionalized tetrahydropyrans†
Abstract
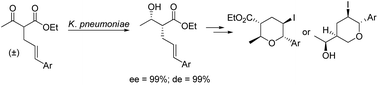
Ketoreductase from growing cells of Klebsiella pneumoniae (NBRC 3319) acts as an efficient reagent for converting racemic α-benzyl/cinnamyl substituted-β-ketoesters to the corresponding β-hydroxy esters with excellent yields and stereoselectivities (ee and de >99 %). The reactions described herein followed a biocatalytic dynamic kinetic reductive resolution (DKRR) pathway, which is reported for the first time with such substrates. It was found that the enzyme system can accept substituted mono-aryl rings with different electronic natures. In addition, it also accepts a substituted naphthyl ring and heteroaryl ring in the α-position of the parent β-ketoester. The synthesized enantiopure β-hydroxy esters were then synthetically manipulated to valuable tetrahydropyran building blocks.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...