 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biochar-augmented biofilters to improve pollutant removal from stormwater – can they improve receiving water quality?†
Alexandria B.
Boehm
 *ab,
Colin D.
Bell
bc,
Nicole J. M.
Fitzgerald
bc,
Elizabeth
Gallo
*ab,
Colin D.
Bell
bc,
Nicole J. M.
Fitzgerald
bc,
Elizabeth
Gallo
 bc,
Christopher P.
Higgins
bc,
Christopher P.
Higgins
 bc,
Terri S.
Hogue
bc,
Terri S.
Hogue
 bc,
Richard G.
Luthy
bc,
Richard G.
Luthy
 ab,
Andrea C.
Portmann
bc,
Bridget A.
Ulrich
d and
Jordyn M.
Wolfand
ab,
Andrea C.
Portmann
bc,
Bridget A.
Ulrich
d and
Jordyn M.
Wolfand
 bce
bce
aDepartment of Civil & Environmental Engineering, Stanford University, Stanford, California, USA 94305. E-mail: aboehm@stanford.edu; Tel: +650 724 9128
bEngineering Research Center (ERC) for Re-inventing the Nation's Urban Water Infrastructure (ReNUWIt), Stanford, California, USA
cCivil and Environmental Engineering, Colorado School of Mines, Golden, Colorado, USA 80401
dNatural Resources Research Institute, University of Minnesota Duluth, Duluth, Minnesota, USA 55811
eCivil Engineering, University of Portland, Portland, Oregon, USA 97203
First published on 13th April 2020
Abstract
Stormwater biofilters are being implemented widely in urban environments to provide green space, alleviate flooding, and improve stormwater quality. However, biofilters with conventional media (sand, soil, and/or mulch or compost) do not reliably remove contaminants from stormwater. Research suggests addition of biochar to the biofilter media can improve the pollutant removal capacity of biofilters. In the current work, we present a systematic review of laboratory and mesocosm studies of biochar-augmented biofilters and an assessment of watershed-scale implementation of biofilters on local water quality. A full text review of 84 papers was conducted; of these, data were extracted from the 14 that met our inclusion criteria. log10 removal of microbial pollutants and trace organic contaminants (TOrCs) by biochar-augmented media is generally greater than those of the controls containing just sand, soil, and/or compost. log10 removal of nitrogen, phosphorous, total organic carbon, and total suspended solids in biochar-augmented biofilters is not clearly higher than those of control experiments. A supplemental analysis of four studies reporting longer-term breakthrough data revealed that TOrC removal effectiveness varies substantially among high temperature wood-based biochars, and that operational lifetimes of full-scale systems constrained by TOrC sorption capacity could range from five months to over seven years depending on the selected biochar. At the watershed-scale, biochar-augmented biofilters can provide enhanced treatment of runoff, resulting in the need for fewer treatment units or a smaller volume of watershed runoff treated to meet water quality criteria compared to their conventional counterparts. While their installation can reduce the load of pollutants to receiving waters, achieving concentration-based water quality targets may prove difficult even when pollutant removal capacity is high. This work highlights the importance of a systems approach to studying how biofilter installation affects water quality within a watershed. We identify several topical areas where further research is needed, especially as installation of biofilters and other stormwater control measures gain popularity in highly urbanized watersheds.
Water impactBiofilters are being implemented widely in urban environments to provide green space, alleviate flooding, and improve stormwater quality. The addition of biochar to biofilter media greatly improves the removal of important stormwater pollutants. However, even widespread installation of biofilters with high pollutant removal may not sufficiently reduce pollutant concentrations in stormwater at the watershed scale. |
Introduction
Traditionally, the main goal of urban stormwater infrastructure is to convey stormwater away from the city as quickly as possible.1 However, a need for sustainable water supply is changing the traditional mindset: harvested stormwater is being viewed as an option for future water supply.2,3 Several major cities have already begun to capture stormwater for urban water supply, though the water is generally used for non-potable activities.4 For example, the city of Berlin, Germany installed a system that collects rooftop runoff from 19 buildings and uses the water for toilet flushing, car washing, and gardens.4 The city of Greater Adelaide, Australia plans is to triple the amount of stormwater harvesting to 60 Mm3 per year to provide additional drinking water for the drought stricken region.2 In the United States, the city of Los Angeles, California uses stormwater and recycled water to recharge its groundwater basins which are subsequently used to supplement drinking water;5 they plan to increase the recharge of stormwater up four times to 239 Mm3 per year.2 Throughout the rest of the of California, stormwater is also considered the most cost-effective source of water for its growing population, after conservation and efficiency measures.6 However, this is not the case in all cities, as stormwater quality and availability are highly variable.3 Thus while stormwater is increasingly being considered as an alternative water source, attention must be given to stormwater pollutants, and creative methods must be developed for reducing their concentrations.7At the same time, stormwater represents one of the greatest sources of pollution to surface waters. The National Stormwater Quality Database (v.4.02 released in January 2015, downloaded from http://www.bmpdatabase.org) contains concentrations of water quality parameters sampled from stormwater outfalls at around 600 sites across the US.8,9 Many of these concentrations exceed US standards and criteria for protecting drinking water, aquatic life, and human health during recreation (Fig. 1).10–12 Further improving the quality of stormwater to enable its use as a water source, particularly when it already is problematic with respect to contaminating surface waters, is a daunting task.
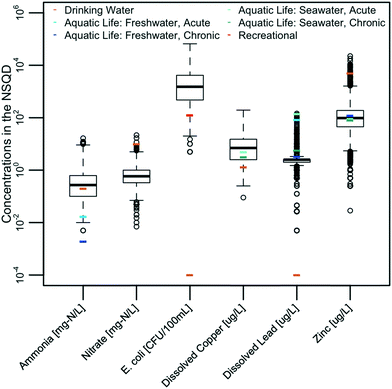 | ||
| Fig. 1 Distribution of concentrations of selected water quality parameters from the National Stormwater Quality Database (NSQD) compared to six federal standards and criteria. Standards with a value of 0 are plotted at 10−4. A majority of concentrations exceed the standards and criteria, highlighting how stormwater quality limits its value as a resource. The water quality parameters were selected based on availability of standards and relevance to the work summarized herein. See Table S1† for further details on the federal standards and criteria. | ||
The effects of stormwater pollutants on surface water quality are varied. Nutrients in urban lawn fertilizer and heavy metals from automobiles are mobilized during wet weather.13–17 In surface waters that receive stormwater, nutrients contribute to eutrophication and metals can be toxic to aquatic life. Runoff can also transport pathogens from human and animal feces to surface waters, which has human and animal health implications.18,19 Additionally, trace organic contaminants (TOrCs), such as plasticizers and pesticides, are present in stormwater, contributing to toxic levels found in surface waters.20–22 While much is unknown about TOrCs in stormwater, work has begun to identify the differences in concentrations across land uses23 and the hydrologic controls over export from watersheds.24
Collectively, contaminated stormwater currently threatens or impairs 2.6 × 108 km (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 miles) of river, 3100 km2 (759
330 miles) of river, 3100 km2 (759![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 483 acres) of lakes, and 43
483 acres) of lakes, and 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2 (106 acres) of estuaries in the US.25 Because stormwater is such a threat to human and ecosystem health, the US government regulates stormwater runoff by issuing discharge permits through the National Pollutant Discharge Elimination System. For a municipality to receive a discharge permit, it must provide a plan for controlling stormwater runoff and water quality. These plans include the implementation of structural stormwater control measures (SCMs), which are engineered landscape depressions that gather, store, infiltrate, evaporate, and/or treat stormwater. SCMs are also referred to as stormwater best management practices (BMPs), low impact development (LID), and green stormwater infrastructure (GSI).26
000 km2 (106 acres) of estuaries in the US.25 Because stormwater is such a threat to human and ecosystem health, the US government regulates stormwater runoff by issuing discharge permits through the National Pollutant Discharge Elimination System. For a municipality to receive a discharge permit, it must provide a plan for controlling stormwater runoff and water quality. These plans include the implementation of structural stormwater control measures (SCMs), which are engineered landscape depressions that gather, store, infiltrate, evaporate, and/or treat stormwater. SCMs are also referred to as stormwater best management practices (BMPs), low impact development (LID), and green stormwater infrastructure (GSI).26
In the US, there are incentives at the national and local levels to promote installation of distributed SCMs, particularly vegetated SCMs that provide urban green space. For example, the USEPA states that they “strongly encourage the use of green infrastructure approaches to manage wet weather”27 and released several memorandums describing their support for using SCMs to achieve US Clean Water Act goals. The European Commission on the environment also promotes the use of SCMs in application of the European Union's international policies.28 China is promoting and testing SCMs to create “sponge cities” for infiltration and retaining stormwater.29 An example of a local incentive to promote distributed stormwater treatment infrastructure can be found in Los Angeles, California. Here, a city ordinance requires any project requiring a building permit to incorporate SCMs into their design plans.30
There are many types of SCMs, which fall on a continuum of design and offer a variety of hydrologic and water quality benefits at different scales, costs, and levels of environmental impact.31 Historically, municipalities and developers implemented larger, regional SCMs like retention ponds, detention basins, and constructed wetlands downstream of pipe networks. In the past two decades, distributed SCMs, such as biofilters, have grown in popularity.
Biofilters are a passive (i.e., require no energy) stormwater treatment technology that consist of an in-ground depression filled with geomedia typically consisting of a mixture of sand, compost, mulch, and/or native soil; they may or may not contain vegetation. Biofilters are also often called bioretention basins or rain gardens; a discussion of regional nomenclature for green infrastructure is provided elsewhere.31 Water infiltrates through to underlying soil or is directed via underdrains to stormwater conveyance systems.7 Typically, a drawdown time of between 12 and 48 hours is required to minimize insect vector concerns.32,33 Internationally, there has been a shift away from centralized basins and media filters toward distributed biofilters, especially since the year 2000 (Fig. 2). This shift toward distributed SCMs may be a result of factors such as lower capital costs, smaller footprints compatible with ultra-urban areas, and co-benefits.31 While the definition and naming convention for different designs of SCMs varies, here we assume that the terms “biofilter” and “bioretention” refer to the same type of SCM, as described above, and use the term “biofilter” throughout.
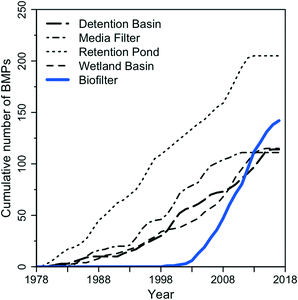 | ||
| Fig. 2 Cumulative number of unique SCMs with water quality data in the International Stormwater BMP Database by year, coded by SCM category. Data were downloaded from http://www.bmpdatabase.org; see Clary et al.139,140 for detailed descriptions of the database. Note that it is assumed that the “Bioretention” category in the database is synonymous with “biofilter” as defined herein. | ||
An additional incentive for installing SCMs, and in particular biofilters, is the assumption that they are able to remove contaminants from stormwater. Conventional biofilters are most effective at removing sediments and particulate-bound pollutants. In fact, biofilters have been shown to reduce concentrations of total suspended solids (TSS),34,35 PAHs,36 and heavy metals34 in stormwater. The removal of dissolved pollutants by biofilters, on the other hand, is more variable. Biofilter effluent concentrations of nitrogen and phosphorus have been reported both at levels greater and less than influent concentrations;34,35,37 nutrient leaching is not uncommon when biofilters are amended with nutrient-rich geomedia such as compost or mulch.38 In addition, soluble TOrCs are expected to have variable removal. For example, while the herbicides atrazine, simazine, and prometryn had event mean concentration reductions ranging from −7 to 58% in field challenge tests for conventional biofilters;39 other TOrCs such as methylthio-benzotriazole (biocide metabolite), tri-n-butylphosphate (plasticizer), and tris-(butoxyethyl) phosphate (plasticizer) were well removed in pilot scale mesocosms, with reductions ranging from 81–98%.40 The removal of microbial contaminants in conventional biofilters is also variable and, as with nutrients, they can be exported from biofilters.41,42 The variable performance of biofilters is further illustrated after analyzing paired influent and effluent data from field-scale biofilters (Fig. 3). In particular, biofilters often export nutrients and fecal indicator bacteria (FIB) while reducing TSS and metals. Collectively, these data suggest that while biofilters, the fastest growing SCM installation (Fig. 2), are promoted by local, state, and US federal regulatory agencies, current designs are not necessarily efficient at removing pollutants from stormwater (Fig. 3). As such, conventional biofilters may not be an ideal solution for achieving water quality goals.
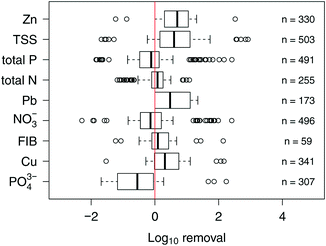 | ||
| Fig. 3 Box and whisker plot showing observed log10 removal efficiencies for select pollutants from conventional biofilters implemented in the field. Data were downloaded from http://www.bmpdatabase.org; see Clary et al.139,140 for detailed descriptions of the database. Positive values indicate a decrease in outflow concentrations relative to inflow, while negative values indicate the opposite. Midline of the box represents the median of observed values; bottom (left) and top (right) of the box represent the 25th and 75th percentiles, respectively. Bottom (left) and top (right) whiskers represent the 10th and 90th percentiles, respectively. Symbols represent data points below and above the 10th and 90th percentiles. Red line indicates no removal. n value provides the number of observations. See ESI† and Table S2 for details on data mining and aggregation methods. | ||
Biofilter performance may be improved in various ways, such as alteration of the hydraulics of the system and creation of a submerged zone,43,44 addition of plants or fungi with pollutant-removal capabilities,45–49 or alteration of geomedia. Though certain types of engineered geomedia have proven effective for targeted contaminant removal (e.g., manganese-oxide, iron-oxide coated sands,50,51 surface-modified biochar,52,53 and functionalized polymer clays54), biochar has shown strong promise for removal of a broad array of stormwater contaminants.55 Biochar is produced by the carbonization of biomass, or the thermal treatment of organic waste such as woodchips in an oxygen-limited environment. This results in a highly porous material that provides high surface area for interaction with various pollutants, making biochar a particularly promising and importantly, a cost-effective amendment for biofilters.
The goal of this review is to summarize work to improve biofilter performance through the augmentation of the filter media with biochar. We also summarize watershed modeling studies that elucidate how efficient distributed biofilters need to be to achieve local water quality objectives. This review is novel in its focus on biochar in the context of stormwater treatment, use of a systematic review, and consideration of work across scales from the laboratory to the watershed. We end with a discussion of needed future work.
Biochar-augmented biofilter performance for reducing pollutant concentrations in stormwater
Methods for systematic review
We conducted a systematic literature review to identify all peer-reviewed studies that used column experiments to investigate the potential for biochar-augmented media to remove contaminants from stormwater following PRISMA guidelines.56 We chose to conduct a systematic review so we would include all papers meeting the inclusion criterion (as described below), and not bias the review by including only papers we were familiar with. As this paper is focused on stormwater, we did not include studies that investigated the removal of contaminants from wastewater57–59 because wastewater and stormwater are not equivalent in their physico-chemical composition, and biofilter design considerations for these applications are distinct. Stormwater tends to have more variable chemistry and microbiology relative to wastewater. Additionally, biofilters are passive, distributed treatment systems that receive intermittent flow, whereas wastewater systems are typically centralized, active, and receive continuous flow.On 10 September 2019, we searched the Web of Science Core Collection (search field = topic), Scopus (search field = title, keyword, abstract) and PubMed (search field = all fields) using the following terms: ((“storm water” OR stormwater OR runoff OR “run-off”) AND biochar*). We combined the search results and removed replicates. We then screened the papers to determine whether they met the following inclusion criteria: (1) the article must be written in English and peer-reviewed, (2) the experiments must be flow-through, column experiments (no batch experiments), (3) the aqueous media must be natural or simulated stormwater and must include dissolved organic material (either naturally present, or added to the simulated matrix), (4) the geomedia in the experimental system must contain unmodified biochar, (5) the geomedia must resemble the geomedia of a conventional bioretention system and contain sand, soil and/or mulch/compost, and (6) the study must report on the removal of a contaminant. Studies that used woodchips and biochar, or surface-modified biochar were not included, as the goal of the review was to characterize how addition of biochar, alone, affected contaminant removal performance across a range of experimental conditions.
The titles and abstracts of the compiled papers were screened by a single researcher to assess whether they could potentially meet our inclusion criteria and if so, they were designated as needing full text review. This part of the review was purposely inclusive. Papers identified for full text review were each screened by two researchers to assess whether they met the inclusion criteria. During full text review, reference lists of review papers were screened for papers to potentially add to the review, and were added if deemed they met our inclusion criteria.
Individual researchers extracted data from all papers that passed our inclusion criteria during full text review. Duplicate data extraction was performed on 25% of these papers by an additional researcher as a QA/QC measure. The following information was extracted from the papers: (1) column dimensions, (2) feedstock, production process, and production temperature of the biochar, (3) composition of geomedia mixture, (4) composition of column eluent, (5) contaminant(s) studied, (6) whether or not the experimental conditions were conducive for biotic processes to contribute to contaminant removal, and (7) log10 removal of contaminants after they passed through the column. If results from control experiments (column experiments with only sand/soil/compost/mulch and no biochar) were also reported, data were extracted for the controls as well. log10 removal data were acquired by (i) extracting the data directly from the text or tables if reported in the article, (ii) calculating log10 removal from the original data if the data were available to the reviewers, or (iii) extracting the data from figures using Plot Digitizer (https://apps.automeris.io/wpd/). For some experiments, chemical contaminant removal was reported as a function of time or volume of water treated. The goal of these studies was to observe ‘breakthrough,’ or when the contaminant concentration in the column effluent passes a specified threshold, indicating that the removal effectiveness of the media is declining. In these cases, the log10 removal data were calculated from the first data points reported after at least three pore volumes had passed through the column: longer-term performance in these systems is further discussed below. For cases where the extracted column effluent concentration was zero, the effluent concentration was replaced with the limit of quantification if reported or the value that corresponded to 99% removal (i.e., 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 removal). Though this approach does not necessarily reflect performance longevity (some log10 removal values were taken at 3 pore volumes while others were taken after over a year of operation), it provides a metric (i.e., log10 removal) for performance comparisons across varying experimental conditions for over a dozen studies.
log10 removal). Though this approach does not necessarily reflect performance longevity (some log10 removal values were taken at 3 pore volumes while others were taken after over a year of operation), it provides a metric (i.e., log10 removal) for performance comparisons across varying experimental conditions for over a dozen studies.
A further analysis was carried out to assess the expected potential duration of the longer-term operational lifetime for removal of chemical contaminants using biochar-augmented media. This analysis was conducted using the four studies from our systematic review54,60–62 that reported results from column experiments that were run long enough to allow breakthrough of chemical contaminants. Data from columns containing activated carbon were also extracted for performance comparison when available.60,61 Values for the volume of water treated prior to breakthrough, the influent contaminant concentration, and the mass of sorbent present in the columns were extracted from the texts. These values were used to calculate a metric for the longevity of chemical contaminant removal performance of the sorbent material: the mass of contaminant removed prior to breakthrough per the mass sorbent present in column (i.e., the chemical removal longevity, in units of μg contaminant per g sorbent). Breakthrough was defined as the first measurement reporting effluent concentrations exceeding 5% of the influent concentration. In cases where breakthrough in sand-only controls (i.e., controls containing no other sorbent material and no biofilm) occurred after greater than three pore volumes,54 the removal for the sorbent-augmented condition was adjusted accordingly to calculate the amount of contaminant removed by the sorbent itself. It is important to note that this metric primarily reflects chemical removal longevity by abiotic, capacity-limited processes such as sorption, and does not reflect the longevity of biological removal processes.
Results of systematic review
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 removal values were extracted from the 14 papers, and these data were used to assess trends in the removal performance. We plotted log10 removal for the biochar-augmented biofilters for each tested contaminant and compared those with the log10 removal of control experiments containing no biochar (Fig. 4, data available from Boehm et al.72).
log10 removal values were extracted from the 14 papers, and these data were used to assess trends in the removal performance. We plotted log10 removal for the biochar-augmented biofilters for each tested contaminant and compared those with the log10 removal of control experiments containing no biochar (Fig. 4, data available from Boehm et al.72).
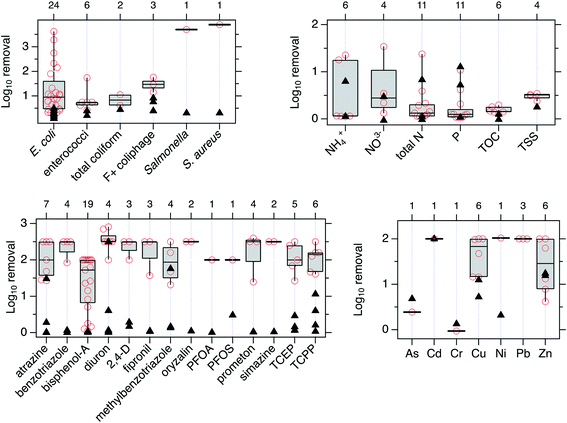 | ||
| Fig. 4 log10 removal of contaminants in biochar-augmented biofilters for different contaminants considered in the 14 papers identified during our systematic review. The red symbols show the log10 removal values for the biochar-containing columns – the number of red symbols for each contaminant is displayed on the top axis. The box and whisker plots show the distribution of log10 removal observed for each contaminant for the biochar-augmented biofilters. The horizontal line in the middle of the box is the median, the top and bottom of the box represent the 75th and 25th percentiles, respectively, the top and bottom whisker represent maximum and minimum log removal. In some cases where few data points were collected, only a subset of these symbols are shown. The solid, black triangle represents the performance observed in all control experiments for the contaminant reported in the same 14 papers. Table S3† defines chemical acronyms. The As and Cr species reported are As(V) and Cr(VI). | ||
Importantly, the data in Fig. 4 are from experiments with diverse experimental designs. For example, experiments in 11 of the 14 (ref. 44, 54, 60, 61, 63–66, 68, 70 and 71) papers were carried out in the laboratory, with the experiments in the 3 remaining papers conducted outside at the mesocosm scale (2 of 14)67,69 or under both laboratory and mesocosm scale (1 of 14).62 Column dimensions, type of biochar, biochar particle size, mass fraction of biochar, and the duration and magnitude of applied stormwater flow rates were distinct across experiments. Additionally, some experiments were conducive to potentially biotic removal mechanisms (by using soils, plants, or natural stormwater that contain microbial communities), while others were carried out under sterile conditions selected to assess abiotic removal mechanisms. Though the distinct experimental design elements were justified for each individual studies' research questions by the respective authors, these inconsistencies make comparison across the studies challenging.
However, despite these variations in experimental design, several clear trends in the log10 removal values emerged. log10 removal of microbial pollutants and TOrCs by biochar-augmented media is generally greater than those of the controls containing just sand, soil, and/or mulch/compost. log10 removal of N-containing nutrients (nitrate, ammonium and total N), phosphorus, total organic carbon, and TSS in biochar-augmented biofilters is similar to those of control experiments. Results are mixed with respect to the metals. Cu, Ni, and Pb show higher log10 removal in biochar-augmented biofilters compared to controls, while log10 removal of As, Cd, Cr, and Zn in biochar-augmented biofilters is similar to or lower than those observed in controls. Data are limited for many pollutants. For example, there have been a relatively large number of experiments with E. coli, but a limited number of experiments with other important microbial pollutants including pathogens. For the TOrCs, more experiments have been conducted with atrazine and bisphenol-A than other chemicals. Similarly, while several experiments were conducted with Cu and Zn, there are limited experiments with other metals.
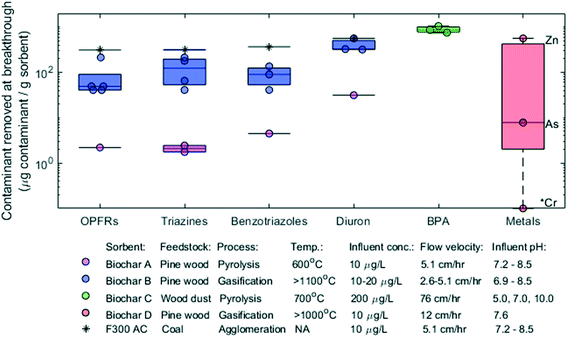 | ||
| Fig. 5 Results for breakthrough data analysis, reported as the mass of contaminant removed at breakthrough (i.e., when the effluent concentration first exceeded 5% of the influent concentration) per mass of sorbent present in the column. Markers show the raw data, boxes encompass the 25th to 75th percentiles, and lines within the boxes represent the median. Markers outside of their corresponding boxes represent statistical outliers, with the exception of the metals for which only three data points were available. Conditions with one reported value (single lines) represent averages of experimental replicates. Relevant experimental details are provided in the legend (flow velocity = volumetric flow rate/column cross sectional area). TOrC subclasses were grouped: the organophosphate flame retardants (OPFRs) are TCPP and TCEP, the triazines are atrazine and prometon, and the benzotriazoles are benzotriazole and methylbenzotriazole. The heavy metals are zinc (Zn), arsenic (As(V)), and chromium (Cr(VI)); markers represent results for the three different metals (labeled accordingly) for a single set of experiments. The activated carbon (AC) is Calgon Filtrasorb™ 300. The asterisk (*) indicates a zero value that cannot be displayed on a log scale. Chemical acronyms are in Table S3.† | ||
Discussion of systematic review
Abiotic removal of metals may occur via complexation, cation-exchange, electrostatic interactions, precipitation, and chemical reduction; the importance of each of these mechanisms heavily depends on the individual metal of interest.74 Considering the log10 removal results in Fig. 4, it appears that biochar may enhance these abiotic removal mechanisms relative to controls for Cu, Ni, and Zn, but not for the other metals. Further, the variability in removal longevity across different metal species shown in Fig. 5 also reflects the understanding that mechanisms for metals removal depend strongly on the characteristics of the metal species.74 Nutrient sorption by biochars has been proposed to be dominated by cation exchange capacity (CEC)75 or co-adsorption with soluble organic matter in the case of ammonia,76 whereas the retention of phosphate likely occurs via precipitation55 or exchange of surface hydroxyl groups.76 Depending on nutrient and biochar properties, nutrient removal efficiencies can be highly variable,77 and some biochars may even be a net source of nutrients (leaching)77,78 as it appears may be occurring for the log10 removal results displayed in Fig. 4. None of the studies that met the review criteria presented nutrient breakthrough data, therefore the longevity for nutrient removal could not be assessed.
It is likely that the main abiotic removal mechanism of TOrCs by high temperature biochars (i.e., those produced by pyrolysis or gasification at temperatures greater than 600 °C) is through adsorption via hydrophobic interactions,60,79 given the hydrophobic nature of neutral TOrCs. The results in Fig. 4 suggest that TOrC removal can be readily improved via the addition of biochar to biofilters, as this was broadly improved relative to sand controls across TOrC classes and biochar types. However, the results in Fig. 5 demonstrate that longevity of TOrC removal depends strongly on biochar type. Though TOrC removal longevity varied widely among biochar types, each type of biochar showed similar performance across all TOrCs evaluated. This is consistent with the understanding that the sorption of uncharged TOrCs to high temperature biochars is dominated by hydrophobic interactions.80 Other factors that may influence TOrC sorption to biochar include TOrC characteristics and water chemistry. For example, greater removal of diuron and benzotriazole relative to the other TOrCs in Fig. 5 may be attributed to reduced steric effects associated with differences in planarity.81 Further, the pH may affect sorption of TOrCs with acidic functional groups, as demonstrated by the breakthrough results for bisphenol-A at different influent pH conditions in Fig. 5. The presence of natural organic matter (NOM) can also significantly reduce TOrC removal longevity due to fouling-induced effects to sorption, though this is not assessed here given that only studies using water containing NOM were considered. Overall, these findings demonstrate that biochar can improve TOrC removal in biochar-augmented biofilters due to enhanced sorption, though the longevity, as defined by the mass of TOrC removed per mass of sorbent will be strongly dependent on the type of biochar.61
While microbial pollutants may be removed via the abiotic processes of filtration and straining,43,55 several lines of evidence also suggest that hydrophobic interactions between microbes and biochar promote their attachment and subsequent filtration,68 which may explain the enhanced removal of microbial pollutants in biochar-augmented biofilters relative to controls in Fig. 4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 removal values extracted, 178 (73%) were from experiments conducted under conditions that were conducive to biotic activity – for example, they either used natural stormwater containing indigenous microbes as the aqueous media, included a plant, or used soil as media. However, those studies were not necessarily designed to investigate whether the inclusion of biochar in the geomedia enhanced biotic versus abiotic removal processes. Nevertheless, information can be gleaned from individual studies in the literature about the potential for biochar to affect biotic contaminant removal processes. For example, the presence of biochar can increase microbial activity, and biochar can facilitate electron transfer owing to its redox-active moieties and aromaticity.82,83 Saquing et al.83 suggest that biochar can function as both electron donor and acceptor, and thus should be seen as a rechargeable reservoir of bioavailable electrons; a feature which could potentially promote contaminant biodegradation. The stimulating effect of biochar on microbial processes has been shown for both Fe(III) minerals reduction84 and nitrate reduction (denitrification).83 The presence of a biofilm in biochar-augmented biofilters can also increase nutrient removal capacity, especially for nitrate and ammonium.44 Biofilm-coated biochar is beneficial for TOrC removal, possibly as a result of microbial biodegradation.60 However, biofilms can reduce microbial pollutant removal, presumably due to decreased surface roughness and less negative surface charge of the biofilm.66 There has been limited work on how biochar might promote inactivation or predation of microbial contaminants, additional modes of possible biotic removal.
log10 removal values extracted, 178 (73%) were from experiments conducted under conditions that were conducive to biotic activity – for example, they either used natural stormwater containing indigenous microbes as the aqueous media, included a plant, or used soil as media. However, those studies were not necessarily designed to investigate whether the inclusion of biochar in the geomedia enhanced biotic versus abiotic removal processes. Nevertheless, information can be gleaned from individual studies in the literature about the potential for biochar to affect biotic contaminant removal processes. For example, the presence of biochar can increase microbial activity, and biochar can facilitate electron transfer owing to its redox-active moieties and aromaticity.82,83 Saquing et al.83 suggest that biochar can function as both electron donor and acceptor, and thus should be seen as a rechargeable reservoir of bioavailable electrons; a feature which could potentially promote contaminant biodegradation. The stimulating effect of biochar on microbial processes has been shown for both Fe(III) minerals reduction84 and nitrate reduction (denitrification).83 The presence of a biofilm in biochar-augmented biofilters can also increase nutrient removal capacity, especially for nitrate and ammonium.44 Biofilm-coated biochar is beneficial for TOrC removal, possibly as a result of microbial biodegradation.60 However, biofilms can reduce microbial pollutant removal, presumably due to decreased surface roughness and less negative surface charge of the biofilm.66 There has been limited work on how biochar might promote inactivation or predation of microbial contaminants, additional modes of possible biotic removal.
Other factors affecting pollutant removal performance
The feedstock and production process affect the resultant biochar's physio-chemical characteristics, including specific surface area, particle size distribution, elemental composition, volatility, and polarity. These properties can affect the removal of stormwater pollutants. For example, higher production temperatures generally lead to biochars characterized by high specific surface area, microporosity, and hydrophobicity – features that are generally associated with efficient removal of TOrCs.86 Low temperature biochars, on the other hand, are more suitable for the removal of inorganic contaminants because they typically contain more polar surface functional groups that can interact with charged or polarizable contaminants such as metals, and promote chemical precipitation and electrostatic attractions.86 Mohanty et al.65 and Abit et al.87 showed biochars produced from woodchip feedstocks effectively removed E. coli from an infiltrating solution in column experiments whereas Sasidharan et al.88 found biochars produced from feedstock of nut shells, rice husk, wheat chaff, a reed, and a mallee did not show effective removal of E. coli and coliphage from a buffer solution. Due to the differences in properties of biochars, it is essential that researchers fully describe and characterize biochars used in their experiments.89 Indeed, some89 have further suggested that well-characterized biochars always be included as part of future studies in the hopes that these materials can be used to benchmark new biochars and enable comparisons across studies.
Association of measurable properties with trends in performance across contaminant classes (and an understanding of the underlying mechanisms that cause such trends) may enable optimization of biochar-based media for comprehensive contaminant removal. For example, as the dominant abiotic removal process for TOrCs is likely adsorption, factors that govern organic contaminant adsorption55 such as surface area, aromaticity, and internal pore size distribution, especially microporosity and mesoporosity61 may be important factors to consider. Presumably for similar reasons, wood-based, high temperature biochars87 with low O/C ratios (low surface polarity, high hydrophobicity) and low volatile matter65 appeared to result in efficient pathogen and indicator organism removal. Of course, as biochar production processes are often not optimized nor managed for generating specific physio-chemical properties, quantitative and predictive performance modeling for biochar-augmented biofilters based solely on physio-chemical characteristics may remain a challenge for the foreseeable future. Indeed, our analysis of chemical removal longevity (Fig. 5) suggests that selection of a biochar based on production characteristics alone is insufficient for predicting pollutant removal from stormwater, and demonstrates the importance of characterizing and testing the selected biochar under operational conditions representative of the intended application location and climate.
Biochar particle size also appears to play an important role in media performance with respect to contaminant removal. Several studies have found that including smaller biochar particle sizes can achieve improved contaminant removal, including bacteria (i.e., E. coli),63 as well as dissolved contaminants such as heavy metals90,91 and organics.92,93 The particle size dependence of dissolved contaminant removal can be attributed to reduced intraparticle kinetic diffusion limitations,61,91 as diffusion paths to internal sorption sites are shorter for smaller particle sizes. Bacteria are too large to diffuse through biochar pores to access internal surface area, so the improved removal of bacteria is likely caused by enhanced accessibility to external surface sites for smaller particle sizes.63 However, smaller particle sizes may also adversely affect hydraulic conditions, which is discussed further below.
It is important to note that addition of biochar to a biofilter may also affect its hydraulic conductivity.55,97 A meta-analysis98 found that amendment of soil with biochar increases saturated hydraulic conductivity by ∼25%. Another study found biochar amendment to increase saturated hydraulic conductivity by 328% in clay-rich soil but decrease by 92% and 67% in sand and organic soils, respectively.99 The effect of biochar addition on hydraulic conductivity of biofilter media will depend on the differences in particle size of the biochar and the sand55,97 and may also depend on the biochar application rate. Addition of biochar to sand media reduced the hydraulic conductivity at higher biochar application rates (>15%),100 and a ten-fold decrease in hydraulic conductivity compared to sand-only was observed by Ray et al.54 when mixing sand (0.6–0.85 mm) with finer biochar particles (0.1–0.3 mm). On the contrary, biochar application rates of 0.5–2% only led to minimal decreases in hydraulic conductivity of a sandy loam soil.101 Given the apparent trade-off between contaminant removal and hydraulic performance, it will be important for practitioners to balance these goals by designing infiltration media to include the minimum biochar particle sizes that allow the desired hydraulic conductivity and infiltration rate.
Watershed scale implementation of biochar-augmented biofilters
The pollutant removal efficiencies of biofilters at the unit scale are promising, but watershed scale efficacy is less well understood. Watershed-scale implementation of SCMs has been investigated regarding water quantity, but fewer studies have investigated the impacts of watershed-scale installation of SCMs on water quality,102,103 and only two studies to date have looked at watershed scale effects of biochar-augmented biofilters.104,105Studies have generally found that installation of biofilters results in improved water quality at the watershed scale, but these results vary in extent depending on watershed size, climate, impervious area treated, and pollutants of concern.102,103 For example, Bedan and Clausen106 monitored a watershed in the US state of Connecticut and found SCM installation decreased storm flows, nitrogen, Pb, and Zn loads compared to a paired control watershed without SCMs, while total P and total suspended solids loads increased relative to the control watershed. Another study monitored a catchment in the city of Melbourne, Australia and found concentrations of suspended sediment, phosphorus, and nitrogen decreased with SCM implementation.107 A statistical analysis of 24 watersheds in the US mid-Atlantic region demonstrated that while watersheds with more SCMs had 48% less total N exports than those with minimal SCMs, there were no differences in phosphorus exports or combined sewer overflows across all watersheds.108 Existing studies show pollutant reduction is often correlated to the hydrologic processes such as infiltration and volume reduction, and not necessarily the biogeochemical processes occurring within the filters.102,104
Surface water quality is regulated at the watershed scale, and biofilters can be used to help reach compliance, but the extent of installation is often substantial and costly. For example, Gagrani et al.109 evaluated the efficacy of using backyard biofilters in conjunction with the existing SCM network to reach mandated pollutant load reductions of 85% for TSS and 70% for total P (in comparison to pre-development conditions) for a watershed in the southeast United States. These authors found that the existing SCM network plus simulated backyard biofilters would result in reductions of only 59% and 51% for TSS and total P, respectively. To reach the mandated pollutant load reductions, at least 70% of the drainage area (including areas already treated with existing SCMs) would need to be routed to additional offline biofilters. This study shows that biofilters can help meet load-based water quality standards, but implementation rates to do so may be impractical.
Similarly, Gallo et al.110 studied urbanized watersheds in Los Angeles and investigated the extent of implementation of SCMs (including biofilters) required to reduce metal loads and meet concentration-based water quality requirements. Computational modeling results show that when SCMs are simulated to capture the 85th percentile storm volume, pollutant loads are greatly reduced (up to 75%, 85%, and 84% in the Ballona Creek, Dominguez Channel, and Los Angeles River watersheds, respectively). However, Gallo et al. found that the reduction of metals to meet concentration-based water quality requirements is difficult to attain. For example, in the Dominguez Channel watershed (184 km2, 71% impervious), despite treating 90% of the watershed runoff with biofilters, Cu only reached 12.5% and Zn only reached 25% compliance.111,112
Given the challenges in meeting water quality requirements with conventional SCMs, Wolfand et al.104 investigated how improving biofilter performance with the addition of biochar may impact water quality at the watershed scale. Implementation of biochar-augmented biofilters was examined using the Ballona Creek watershed as a case study. The tradeoffs between number of biofilters, percent of the watershed runoff treated, infiltrative properties, and biofilter removal efficiency on downstream receiving water quality was quantified. Conventional biofilters average about 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 or 55% removal of FIB. Biochar-augmented biofilters have been shown to increase the removal of FIB in runoff to upwards of 3.5
log10 or 55% removal of FIB. Biochar-augmented biofilters have been shown to increase the removal of FIB in runoff to upwards of 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10, depending on the type of biochar and concentration of natural organic matter in stormwater65 (Fig. 4). A coupled hydrologic water quality model was used to show that these biochar-augmented biofilters can provide significant reduction of FIB load. For example, just a 0.25
log10, depending on the type of biochar and concentration of natural organic matter in stormwater65 (Fig. 4). A coupled hydrologic water quality model was used to show that these biochar-augmented biofilters can provide significant reduction of FIB load. For example, just a 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 increase in removal efficiency could reduce bacterial load by 29%. Assuming biochar results in 3
log10 increase in removal efficiency could reduce bacterial load by 29%. Assuming biochar results in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 efficiency, treating the entire watershed with 25
log10 efficiency, treating the entire watershed with 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 enhanced biofilters would result in a 100% reduction in FIB load while conventional biofilters would result in only 62% reduction (Fig. 6). Results for reducing FIB concentration are, however, less promising. Because FIB concentrations can vary by orders of magnitude, unless 100% of runoff is treated by SCMs, it is impossible to reduce concentrations to below recreational contact standards. Water bypassing SCMs mixes with water treated by SCMs to push concentrations above recreational contact standards (even when 95% of watershed runoff is treated). Regardless, biochar-augmented biofilters show promise for reducing FIB load at the watershed scale.
000 enhanced biofilters would result in a 100% reduction in FIB load while conventional biofilters would result in only 62% reduction (Fig. 6). Results for reducing FIB concentration are, however, less promising. Because FIB concentrations can vary by orders of magnitude, unless 100% of runoff is treated by SCMs, it is impossible to reduce concentrations to below recreational contact standards. Water bypassing SCMs mixes with water treated by SCMs to push concentrations above recreational contact standards (even when 95% of watershed runoff is treated). Regardless, biochar-augmented biofilters show promise for reducing FIB load at the watershed scale.
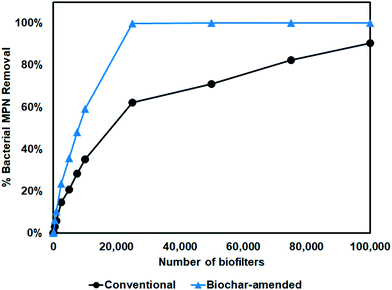 | ||
Fig. 6 Simulated annual bacterial load reduction near outlet of the Ballona Creek watershed (Los Angeles, CA). Conventional biofilters are assumed to provide 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 removal while biochar-augmented filters are assumed to provide 3 log10 removal while biochar-augmented filters are assumed to provide 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 removal (Fig. 4). Biofilters are unlined (infiltrating) and treat 100% of the watershed runoff. log10 removal (Fig. 4). Biofilters are unlined (infiltrating) and treat 100% of the watershed runoff. | ||
One important takeaway from this work is that amending filters with biochar can reduce the number of biofilters needed to meet a water quality goal or standard. For example, in the Ballona Creek watershed, to meet a target of 80% FIB load reduction, about 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 conventional biofilters are required. If biochar-augmented biofilters are installed, however, only approximately 20
000 conventional biofilters are required. If biochar-augmented biofilters are installed, however, only approximately 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 biofilters would be required. Given the median capital cost of a biofilter in Los Angeles is $14.60 per unit,111 this could represent significant cost savings to a municipality; in this case, approximate capital cost would decrease four-fold from $1.2m to $290
000 biofilters would be required. Given the median capital cost of a biofilter in Los Angeles is $14.60 per unit,111 this could represent significant cost savings to a municipality; in this case, approximate capital cost would decrease four-fold from $1.2m to $290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
A case study in the city of San Diego in the US was conducted to demonstrate how watershed-scale implementation of biochar-augmented biofilters may help reduce pesticide concentrations in the San Diego River.105 Laboratory-scale work indicates biochar-augmented biofilters can greatly reduce concentrations of TOrCs (Fig. 4). While rarely regulated, pyrethroid pesticides as well as fipronil are increasingly detected in urban stormwater and subsequently urban waterways.22,105,113–115 Results show that installation of infiltrating biochar-augmented biofilters could reduce loads of bifenthrin, a common pyrethroid, by 93%; the same conventional biofilters provide 78% bifenthrin load reduction. This result was even more exaggerated for fipronil, which is more hydrophilic and thus even more poorly removed by conventional biofilters (reduction of 93% with biochar compared to 55% for conventional). In the same case study,105 in-river pesticide concentrations were more sensitive to biofilter performance than pesticide load, particularly when the biofilters were unable to infiltrate into the subsurface. In this case, installation of conventional biofilters throughout the watershed was unable to reduce concentrations to below concentration-based aquatic toxicity benchmarks. Switching to biochar-augmented filters resulted in significant concentration reductions; during a simulated storm event, concentrations of bifenthrin were below toxicity benchmarks 46% of the time and fipronil concentrations were below benchmarks 97% of the time.
Both watershed-scale studies (in Los Angeles and San Diego) are examples of where biochar-augmented biofilters were predicted to either improve water quality enough to meet load-based standards/criteria or be used to reduce the number of filters needed overall. Adding biochar may ensure that water quality benefits are achieved not only by the hydrologic processes of infiltration and volume reduction but also by the biogeochemical processes within the filter.
Discussion and future research
Research needs at the watershed scale
Biochar-augmented biofilters have shown great promise in the laboratory and mesocosm-scale for removing pollutants from stormwater. However, there is still a need for empirical studies at the field and watershed scales to verify their long-term performance. Considerations at these scales include dynamic hydrologic and water quality loads as well as imperfect installation and maintenance. Empirical field studies must go beyond monitoring and include experimental controls that allow for explicit comparisons to be made between sites with and without biofilters, as well as between sites with biochar-augmented versus conventional biofilters. Studies should be conducted in different regions to account for climatic variations like monsoonal precipitation, freeze/thaw cycles, and prolonged dry conditions. Additionally, they should be executed over long periods of time (months to years) to identify pollutant breakthrough and other declines in performance.Few studies have specifically examined how the presence of multiple contaminants in stormwater affect the performance of biochar-augmented biofilters. Co-existing contaminants in a realistic stormwater matrix could either limit adsorption through competition for sorption sites (e.g. Pb and Cr suppress atrazine retention on biochar, but not vice versa116) or enhance overall removal via precipitation55 or co-adsorption.76 Furthermore, competitive adsorption among metals can play an important role; for example Park et al.117 reported that Cd and Cu can easily compete with Zn during sorption to biochar. Overall, research on biofilter performance considering interactive processes occurring with contaminant mixtures or under field conditions is limited and additional research is warranted.
Results of empirical studies should then inform numerical watershed models, which can evaluate biochar-augmented biofilters at larger spatial scales and under future scenarios of climate change and development. The current modeling approaches are limited. For example, some popular stormwater modeling software packages ignore water quality treatment in biofilters altogether (e.g. EPA-SWMM, see Rossman118). Others use simple, lumped treatment algorithms such as the removal efficiency model (i.e., log-removal) or a first order decay rate.119 Explicit modeling of the unit processes in biofilters described above could improve simulation and allow for more nuanced scenario testing. Additionally, numerical models should be expanded to include changes in treatment performance through time and in response to maintenance activities such as vegetation removal or media replacement and flushing.120 In addition, empirical studies of contaminant removal should report log10-removal of their contaminant or removal rate constants so that contaminant removal can be parameterized in applicable watershed models. While time until contaminant breakthrough is commonly reported in studies of chemical removal by columns of geomedia, that information is not useful in the context of the watershed modeling described above.
Water quality models can be an integral tool in understanding tradeoffs in watershed-scale management approaches. Water quality is impacted and often regulated at the watershed scale, so understanding and planning for management decisions within the entire watershed is more impactful than responding to water quality issues at a single site. Wolfand et al.104,105 and Gallo et al.111 concluded that the end watershed management goal greatly impacts the optimal spatial distribution, size, infiltrative properties, and performance needs of biofilters (Table 1). For example, concentration-based versus load-based standards should be approached differently; biochar-augmented non-infiltrating filters are recommended to meet concentration-based standards whereas load-based standards are best met with infiltrating conventional biofilters (Table 1).
| Goal | Infiltrating? | Enhanced/conventional? |
|---|---|---|
| Maintain high quality environmental flows | No | Enhanced |
| Increase groundwater recharge | Yes | Either |
| Maximize water available for reuse | No | Either |
| Meet concentration-based water quality standards | No | Enhanced |
| Meet load-based water quality standards | Yes | Either |
| Reduce flooding | Yes | Either |
Social and economic considerations
There is potential for biofilters, in general, to provide urban communities with co-benefits, defined here as additional benefits beyond stormwater quantity and quality control. When vegetated, biofilters add green space to urban environments, contributing to a broader network of green infrastructure which includes street trees, parks, wetlands, and green roofs. A meta-analysis98 indicated that addition of biochar to soils can increase available water holding capacity by 15%; which in turn can promote plant growth62,121 and plant survival during drought.122 The latter will be increasingly important if biofilters are perceived as greenspace in increasingly water-stressed climates.Research on human health and well-being co-benefits provided specifically by stormwater systems such as biofilters is limited.123 However, the co-benefits of green spaces, in general, have been well studied.124 For example, urban trees improve air quality by removing particulates and combat climate change by sequestering carbon.125,126 Domestic gardens reduce local temperatures and reduce building energy consumption.127 Green space can also provide physical and mental health benefits.128 It is logical to extrapolate these findings from other urban green spaces to vegetated biofilters. However, the co-benefits are unlikely to be realized if the biofilters are unvegetated, as is the case for much of the SCMs installed in the US city of Philadelphia.129 The co-benefits of biochar-augmented biofilters are unknown, but it is likely they provide similar if not more co-benefits than conventional biofilters because of improved plant health, assuming they occupy the same footprint and have similar planting schemes.
Biochar costs vary widely, depending on the feedstock, transport, pyrolysis operation, and other factors, but are estimated to range from $350 to $18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per metric ton.130,131 The current cost of biochar is significant compared to construction sand, which is typically used as biofilter media and cost about $8.6 per metric ton in 2015.132 However, the incremental cost of adding biochar may be worthwhile when also considering its water quality benefits. As noted above, widescale adoption of biochar-augmented biofilters in a watershed may reduce the number of filters needed to comply with water quality standards, thus reducing the overall cost of infrastructure. This tradeoff in material cost and number of units needed requires more study.
000 per metric ton.130,131 The current cost of biochar is significant compared to construction sand, which is typically used as biofilter media and cost about $8.6 per metric ton in 2015.132 However, the incremental cost of adding biochar may be worthwhile when also considering its water quality benefits. As noted above, widescale adoption of biochar-augmented biofilters in a watershed may reduce the number of filters needed to comply with water quality standards, thus reducing the overall cost of infrastructure. This tradeoff in material cost and number of units needed requires more study.
It is expected that biochar will become more economically competitive as the biochar market develops and carbon pricing is more common. Biochar provides carbon sequestration benefits, so the cost of biochar may even be net zero or negative if the feedstock is a waste product (yard clippings, agricultural waste, spent grain) under a carbon pricing regime. This contrasts with activated carbon, which may be similar in price to biochar, but typically has a positive carbon dioxide demand.130 In fact, Roberts et al.133 note that the breakeven cost of biochar produced with yard waste feedstock occurs when carbon is priced at $2 CO2e per metric ton. Carbon is currently priced at about $15 CO2e per metric ton in the US state of California and averaged about $4.40 CO2e per metric ton in the nine northeastern US states that participate in the Regional Greenhouse Gas Initiative in 2018.134
System performance considerations and technology diffusion
The lifetime of the biochar-augmented media is another remaining knowledge gap. As biological removal processes can be more sustainably maintained over long-term operation than capacity-limited abiotic sorption processes, most work on this topic has focused on prediction of media exhaustion times for contaminants removed by abiotic processes. Davis et al.135 used results from pilot-scale experiments to predict that the lifetime of conventional biofilters would be limited to 15 years based on accumulation of non-degradable metals, a result which could be extended to biochar-augmented biofilters that similarly or more effectively capture metals than conventional systems. While it remains unclear if TOrC removal can be maintained over this duration, several studies have predicted that TOrC removal can be maintained for over 10 years if biodegradation can contribute to TOrC removal in well-designed systems.60,61,136 Considering the breakthrough results (Fig. 5) and the sizing and operational assumptions from Ulrich et al.,67 expected exhaustion times for systems with high temperature wood-based biochars could range from about five months (for biochar A, pyrolysis at 600 °C) to over seven years (for biochar B, gasification). Therefore, testing the selected biochar for removal of the targeted contaminants will be essential to ensure sorption capacity can be maintained.While media composition can be manipulated to maximize sorption capacity, our understanding of the effects of weathering under actual environmental conditions on long-term performance degradation is particularly lacking. In particular, extreme flow conditions and freeze/thaw cycles can adversely affect performance, and clogging137 or channeling138 can cause systems to fail long before media exhaustion is reached. Therefore, field studies that monitor long-term performance are warranted. Moreover, as there is currently a disconnect between our understanding of the contaminant removal processes at the laboratory scale versus the field scale, future work should seek to bridge this gap by incorporating current knowledge into design considerations and monitoring system performance over multiple years. This will require interdisciplinary efforts among researchers in chemistry, biology, and hydrology, as well as collaborations between researchers and practitioners to ensure effective diffusion of this technology into broader practice.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work received funding from NSF through grant number EEC-1028968, NSF = US National Science Foundation (http://www.nsf.gov/). The sponsor played no role in the study design, data collection and analysis, decision to publish or prep of the manuscript. This work was also partially supported under Assistance Agreement No. R836174 awarded by the U.S. EPA to the Colorado School of Mines, the Nature Conservancy, and the University of California-Berkeley. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the agency. EPA does not endorse any products or commercial services mentioned in this publication.References
- A. H. Roy, S. J. Wenger, T. D. Fletcher, C. J. Walsh, A. R. Ladson, W. D. Shuster, H. W. Thurston and R. R. Brown, Impediments and Solutions to Sustainable, Watershed-Scale Urban Stormwater Management: Lessons from Australia and the United States, Environ. Manage., 2008, 42(2), 344–359, DOI:10.1007/s00267-008-9119-1.
- R. G. Luthy, S. Sharvelle and P. Dillon, Urban Stormwater to Enhance Water Supply, Environ. Sci. Technol., 2019, 53(10), 5534–5542, DOI:10.1021/acs.est.8b05913.
- W. Wu, G. C. Dandy, H. R. Maier and S. Maheepala, Identification of Optimal Water Supply Portfolios for a Major City, J. Water Resour. Plan Manag., 2017, 143(9), 05017007 CrossRef.
- S. M. Hamdan, A Literature Based Study of Stormwater Harvesting as a New Water Resource, Water Sci. Technol., 2009, 60(5), 1327–1339, DOI:10.2166/wst.2009.396.
- Sanitation Districts of Los Angeles, Water Reuse Program https://www.lacsd.org/waterreuse/ (accessed Dec 1, 2019).
- H. Cooley, R. Phurisamban and P. Gleick, The Cost of Alternative Urban Water Supply and Efficiency Options in California, Environ. Res. Commun., 2019, 1(4), 042001, DOI:10.1088/2515-7620/ab22ca.
- J. E. Grebel, S. K. Mohanty, A. A. Torkelson, A. B. Boehm, C. P. Higgins, R. M. Maxwell, K. L. Nelson and D. L. Sedlak, Engineered Infiltration Systems for Urban Stormwater Reclamation, Environ. Eng. Sci., 2013, 30(8), 437–454, DOI:10.1089/ees.2012.0312.
- R. Pitt, A. Maestre and J. Clary, The National Stormwater Quality Database (NSQD), Version 4.02, 2018 Search PubMed.
- R. Pitt, A. Maestre and R. Morquecho, The National Stormwater Quality Database (NSQD, Version 1.1), 2004 Search PubMed.
- USEPA (United States Environmental Protection Agency), National Primary Drinking Water Regulations, https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations Search PubMed.
- USEPA (United States Environmental Protection Agency), National Recommended Water Quality Criteria - Aquatic Life Criteria Tablehttps://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table Search PubMed.
- USEPA (United States Environmental Protection Agency), Recreational Water Quality Criteria, https://www.epa.gov/wqc/2012-recreational-water-quality-criteria, 2012 Search PubMed.
- E. S. Bernhardt, L. E. Band, C. J. Walsh and P. E. Berke, Understanding, Managing, and Minimizing Urban Impacts on Surface Water Nitrogen Loading, Ann. N. Y. Acad. Sci., 2008, 1134(1), 61–96 CrossRef CAS PubMed.
- W. Muschack, Pollution of Street Run-off by Traffic and Local Conditions, Sci. Total Environ., 1990, 93, 419–431, DOI:10.1016/0048-9697(90)90133-F.
- J. N. Brown and B. M. Peake, Sources of Heavy Metals and Polycyclic Aromatic Hydrocarbons in Urban Stormwater Runoff, Sci. Total Environ., 2006, 359, 145–155, DOI:10.1016/j.scitotenv.2005.05.016.
- P. D. La Valle, Domestic Sources of Stream Phosphates in Urban Streams, Water Res., 1975, 9(10), 913–915, DOI:10.1016/0043-1354(75)90041-X.
- R. J. Waschbusch, W. R. Selbig and R. T. BannermanSources of Phosphorus in Stormwater and Street Dirt from Two Urban Residential Basins in Madison. Wisconsin, 1994, vol. 95, p. 1999 Search PubMed.
- S. C. Jiang, K.-Y. Lim, X. Huang, D. McCarthy and A. J. Hamilton, Human and Environmental Health Risks and Benefits Associated with Use of Urban Stormwater, Wiley Interdiscip. Rev.: Water, 2015, 2(6), 683–699, DOI:10.1002/wat2.1107.
- M. A. Miller, I. A. Gardner, C. Kreuder, D. M. Paradies, K. R. Worcester, D. A. Jessup, E. Dodd, M. D. Harris, J. A. Ames and A. E. Packham, et al., Coastal Freshwater Runoff Is a Risk Factor for Toxoplasma Gondii Infection of Southern Sea Otters (Enhydra Lutris Nereis), Int. J. Parasitol., 2002, 32(8), 997–1006, DOI:10.1016/S0020-7519(02)00069-3.
- R. Pitt, K. Goodson, O. Ogburn, V. Eppakayala, S. U. Veeravalli, J. Bathi, B. Wilson, S. Subramaniam and S. Clark, Development, O. of R. and. Identification and Treatment of Emerging Contaminants in Wet Weather Flows, 2013, No. EP-C-07-014 Search PubMed.
- P. M. Bradley, C. A. Journey, K. M. Romanok, L. B. Barber, H. T. Buxton, W. T. Foreman, E. T. Furlong, S. T. Glassmeyer, M. L. Hladik and L. R. Iwanowicz, et al., Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in U.S. Streams, Environ. Sci. Technol., 2017, 51(9), 4792–4802, DOI:10.1021/acs.est.7b00012.
- J. R. Masoner, D. W. Kolpin, I. M. Cozzarelli, L. B. Barber, D. S. Burden, W. T. Foreman, K. J. Forshay, E. T. Furlong, J. F. Groves and M. L. Hladik, et al. Urban Stormwater: An Overlooked Pathway of Extensive Mixed Contaminants to Surface and Groundwaters in the United States, Environ. Sci. Technol., 2019, 53(17), 10070–10081, DOI:10.1021/acs.est.9b02867.
- A. Burant, W. Selbig, E. T. Furlong and C. P. Higgins, Trace Organic Contaminants in Urban Runoff: Associations with Urban Land-Use, Environ. Pollut., 2018, 242, 2068–2077, DOI:10.1016/j.envpol.2018.06.066.
- J. A. Brown, C. D. Bell, T. S. Hogue, C. P. Higgins and W. R. Selbig, An Integrated Statistical and Deterministic Hydrologic Model for Analyzing Trace Organic Contaminants in Commercial and High-Density Residential Stormwater Runoff, Sci. Total Environ., 2019, 673, 656–667, DOI:10.1016/j.scitotenv.2019.03.327.
- USEPA (United States Environmental Protection Agency), National Summary of State Information in the Assessment and Total Maximum Daily Load Tracking and Implementation System (ATTAINS), 2019 Search PubMed.
- T. D. Fletcher, W. Shuster, W. F. Hunt, R. Ashley, D. Butler, S. Arthur, S. Trowsdale, S. Barraud, A. Semadeni-Davies and J.-L. Bertrand-Krajewski, et al. SUDS, LID, BMPs, WSUD and More – The Evolution and Application of Terminology Surrounding Urban Drainage, Urban Water J., 2015, 12(7), 525–542, DOI:10.1080/1573062X.2014.916314.
- USEPA. https://www.epa.gov/green-infrastructure/integrating-green-infrastructure-federal-regulatory-programs (accessed Dec 1, 2019).
- European Commission. The EU Strategy on Green Infrastructure https://ec.europa.eu/environment/nature/ecosystems/strategy/index_en.htm (accessed Dec 1, 2019).
- C. Zevenbergen, D. Fu and A. Pathirana, Transitioning to Sponge Cities: Challenges and Opportunities to Address Urban Water Problems in China, Water, 2018, 10(9), 1230, DOI:10.3390/w10091230.
- City of LA Environment and Sanitation. ORDINANCE AND HISTORY https://www.lacitysan.org/san/faces/home/portal/s-lsh-wwd/s-lsh-wwd-wp/s-lsh-wwd-wp-lid/s-lsh-wwd-wp-lid-oh?_adf.ctrl-state=u6fw48wmy_5&_afrLoop=16478971577690868#! (accessed Dec 1, 2019).
- C. D. Bell, K. Spahr, E. Grubert, J. Stokes-Draut, E. Gallo, J. E. Mccray and T. S. Hogue, Decision Making on the Gray-Green Stormwater Infrastructure Continuum, J. Sustain. Water Built Environ., 2019, 5(1), 0000871, DOI:10.1061/JSWBAY.0000871.
- Urban Storm Drainage Criteria Manual. Volume 3: Stormwater Quality, Urban Drainage Flood Control District (UDFCD), 2015 Search PubMed.
- Development Best Management Practices Handbook: Low Impact Development Manual, City of Los Angeles, 2011 Search PubMed.
- B. E. Hatt, T. D. Fletcher and A. Deletic, Hydrologic and Pollutant Removal Performance of Stormwater Biofiltration Systems at the Field Scale, J. Hydrol., 2009, 365(3), 310–321, DOI:10.1016/j.jhydrol.2008.12.001.
- T. Lucke and P. W. B. Nichols, The Pollution Removal and Stormwater Reduction Performance of Street-Side Bioretention Basins after Ten Years in Operation, Sci. Total Environ., 2015, 536, 784–792, DOI:10.1016/j.scitotenv.2015.07.142.
- C. J. DiBlasi, H. Li, A. P. Davis and U. Ghosh, Removal and Fate of Polycyclic Aromatic Hydrocarbon Pollutants in an Urban Stormwater Bioretention Facility, Environ. Sci. Technol., 2009, 43(2), 494–502, DOI:10.1021/es802090g.
- M. E. Dietz and J. C. Clausen, A Field Evaluation of Rain Garden Flow and Pollutant Treatment, Water, Air, Soil Pollut., 2005, 167(1), 123–138, DOI:10.1007/s11270-005-8266-8.
- G. H. LeFevre, K. H. Paus, P. Natarajan, J. S. Gulliver, P. J. Novak and R. M. Hozalski, Review of Dissolved Pollutants in Urban Storm Water and Their Removal and Fate in Bioretention Cells, J. Environ. Eng., 2015, 141(1), 04014050, DOI:10.1061/(ASCE)EE.1943-7870.0000876.
- K. Zhang, V. Valognes, D. Page, A. Deletic and D. McCarthy, Validation of Stormwater Biofilters Using In-Situ Columns, Sci. Total Environ., 2016, 544, 48–55, DOI:10.1016/j.scitotenv.2015.11.150.
- K. Bester and D. Schäfer, Activated Soil Filters (Bio Filters) for the Elimination of Xenobiotics (Micro-Pollutants) from Storm- and Waste Waters, Water Res., 2009, 43(10), 2639–2646, DOI:10.1016/j.watres.2009.03.026.
- J. M. Hathaway, W. F. Hunt, A. K. Graves and J. D. Wright, Field Evaluation of Bioretention Indicator Bacteria Sequestration in Wilmington, North Carolina, J. Environ. Eng., 2011, 137(12), 1103–1113, DOI:10.1061/(ASCE)EE.1943-7870.0000444.
- L. Zhang, E. A. Seagren, A. P. Davis and J. S. Karns, Effects of Temperature on Bacterial Transport and Destruction in Bioretention Media: Field and Laboratory Evaluations, Water Environ. Res., 2012, 84(6), 485–496, DOI:10.2175/106143012X13280358613589.
- M. A. Rippy, Meeting the Criteria: Linking Biofilter Design to Fecal Indicator Bacteria Removal, Wiley Interdiscip. Rev.: Water, 2015, 2(5), 577–592, DOI:10.1002/wat2.1096.
- A. R. M. N. Afrooz and A. B. Boehm, Effects of Submerged Zone, Media Aging, and Antecedent Dry Period on the Performance of Biochar-Amended Biofilters in Removing Fecal Indicators and Nutrients from Natural Stormwater, Ecol. Eng., 2017, 102, 320–330, DOI:10.1016/j.ecoleng.2017.02.053.
- A. Taylor, A. Flatt, M. Beutel, M. Wolff, K. Brownson and P. Stamets, Removal of Escherichia Coli from Synthetic Stormwater Using Mycofiltration, Ecol. Eng., 2014, 78, 79–86, DOI:10.1016/j.ecoleng.2014.05.016.
- J. M. Wolfand, G. H. LeFevre and R. G. Luthy, Metabolization and Degradation Kinetics of the Urban-Use Pesticide Fipronil by White Rot Fungus Trametes Versicolor, Environ. Sci.: Processes Impacts, 2016, 18(10), 1256–1265, 10.1039/C6EM00344C.
- A. Taylor, J. Wetzel, E. Mudrock, K. King, J. Cameron, J. Davis and J. McIntyre, Engineering Analysis of Plant and Fungal Contributions to Bioretention Performance, Water, 2018, 10(9), 1226, DOI:10.3390/w10091226.
- J. C. Pritchard, Y.-M. Cho, N. Ashoori, J. M. Wolfand, J. Sutton, M. Carolan, E. Gamez Jr., K. Doan, J. S. Wiley and R. G. Luthy, Benzotriazole Uptake and Removal in Vegetated Biofilter Mesocosms Planted with Carex Praegracilis, Water, 2018, 10(11), 1606, DOI:10.20944/preprints201810.0007.v1.
- C. P. Muerdter, C. K. Wong and G. H. Lefevre, Emerging Investigator Series: The Role of Vegetation in Bioretention for Stormwater Treatment in the Built Environment: Pollutant Removal, Hydrologic Function, and Ancillary Benefits, Environ. Sci.: Water Res. Technol., 2018, 4(5), 592–612, 10.1039/c7ew00511c.
- J. E. Grebel, J. A. Charbonnet and D. L. Sedlak, Oxidation of Organic Contaminants by Manganese Oxide Geomedia for Passive Urban Stormwater Treatment Systems, Water Res., 2016, 88, 481–491, DOI:10.1016/j.watres.2015.10.019.
- S. K. Mohanty, A. A. Torkelson, H. Dodd, K. L. Nelson and A. B. Boehm, Engineering Solutions to Improve the Removal of Fecal Indicator Bacteria by Bioinfiltration Systems during Intermittent Flow of Stormwater, Environ. Sci. Technol., 2013, 47(19), 10791–10798, DOI:10.1021/es305136b.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo and M. Chen, Progress in the Preparation and Application of Modified Biochar for Improved Contaminant Removal from Water and Wastewater, Bioresour. Technol., 2016, 214, 836–851, DOI:10.1016/j.biortech.2016.05.057.
- A. Y. T. Lau, D. C. W. Tsang, N. J. D. Graham, Y. S. Ok, X. Yang and X. Li, Surface-Modified Biochar in a Bioretention System for Escherichia Coli Removal from Stormwater, Chemosphere, 2017, 169, 89–98, DOI:10.1016/j.chemosphere.2016.11.048.
- J. R. Ray, I. A. Shabtai, M. Teixidó, Y. G. Mishael and D. L. Sedlak, Polymer-Clay Composite Geomedia for Sorptive Removal of Trace Organic Compounds and Metals in Urban Stormwater, Water Res., 2019, 157, 454–462, DOI:10.1016/j.watres.2019.03.097.
- S. K. Mohanty, R. Valenca, A. W. Berger, I. K. M. Yu, X. Xiong, T. M. Saunders and D. C. W. Tsang, Plenty of Room for Carbon on the Ground: Potential Applications of Biochar for Stormwater Treatment, Sci. Total Environ., 2018, 625, 1644–1658, DOI:10.1016/j.scitotenv.2018.01.037.
- D. Moher, A. Liberati, J. Tetzlaff and D. G. Altman, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement, PLoS Med., 2009, 6(7), e1000097, DOI:10.1371/journal.pmed.1000097.
- T. M. Huggins, A. Haeger, J. C. Biffinger and Z. J. Ren, Granular Biochar Compared with Activated Carbon for Wastewater Treatment and Resource Recovery, Water Res., 2016, 94, 225–232, DOI:10.1016/j.watres.2016.02.059.
- K. A. Thompson, K. K. Shimabuku, J. P. Kearns, D. R. U. Knappe, R. S. Summers and S. M. Cook, Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment, Environ. Sci. Technol., 2016, 50(20), 11253–11262, DOI:10.1021/acs.est.6b03239.
- N. A. Qambrani, Md. M. Rahman, S. Won, S. Shim and C. Ra, Biochar Properties and Eco-Friendly Applications for Climate Change Mitigation, Waste Management, and Wastewater Treatment: A Review, Renewable Sustainable Energy Rev., 2017, 79, 255–273, DOI:10.1016/j.rser.2017.05.057.
- B. A. Ulrich, M. Vignola, K. Edgehouse, D. Werner and C. P. Higgins, Organic Carbon Amendments for Enhanced Biological Attenuation of Trace Organic Contaminants in Biochar-Amended Stormwater Biofilters, Environ. Sci. Technol., 2017, 51(16), 9184–9193, DOI:10.1021/acs.est.7b01164.
- B. A. Ulrich, E. A. Im, D. Werner and C. P. Higgins, Biochar and Activated Carbon for Enhanced Trace Organic Contaminant Retention in Stormwater Infiltration Systems, Environ. Sci. Technol., 2015, 49(10), 6222–6230, DOI:10.1021/acs.est.5b00376.
- L. Lu and B. Chen, Enhanced Bisphenol A Removal from Stormwater in Biochar-Amended Biofilters: Combined with Batch Sorption and Fixed-Bed Column Studies, Environ. Pollut., 2018, 243, 1539–1549, DOI:10.1016/j.envpol.2018.09.097.
- S. K. Mohanty and A. B. Boehm, Escherichia Coli Removal in Biochar-Augmented Biofilter: Effect of Infiltration Rate, Initial Bacterial Concentration, Biochar Particle Size, and Presence of Compost, Environ. Sci. Technol., 2014, 48(19), 11535–11542, DOI:10.1021/es5033162.
- S. K. Mohanty and A. B. Boehm, Effect of Weathering on Mobilization of Biochar Particles and Bacterial Removal in a Stormwater Biofilter, Water Res., 2015, 85, 208–215, DOI:10.1016/j.watres.2015.08.026.
- S. K. Mohanty, K. B. Cantrell, K. L. Nelson and A. B. Boehm, Efficacy of Biochar to Remove Escherichia Coli from Stormwater under Steady and Intermittent Flow, Water Res., 2014, 61, 288–296, DOI:10.1016/j.watres.2014.05.026.
- A. R. M. N. Afrooz and A. B. Boehm, Escherichia Coli Removal in Biochar-Modified Biofilters: Effects of Biofilm, PLoS One, 2016, 11(12), e0167489 CrossRef PubMed.
- B. A. Ulrich, M. Loehnert and C. P. Higgins, Improved Contaminant Removal in Vegetated Stormwater Biofilters Amended with Biochar, Environ. Sci.: Water Res. Technol., 2017, 3, 726–734, 10.1039/C7EW00070G.
- A. R. M. N. Afrooz, A. K. Pitol, D. Kitt and A. B. Boehm, Role of Microbial Cell Properties on Bacterial Pathogen and Coliphage Removal in Biochar-Modified Stormwater Biofilters, Environ. Sci.: Water Res. Technol., 2018, 4(12), 2160–2169, 10.1039/C8EW00297E.
- B. P. Kranner, A. R. M. N. Afrooz, N. J. M. Fitzgerald and A. B. Boehm, Fecal Indicator Bacteria and Virus Removal in Stormwater Biofilters: Effects of Biochar, Media Saturation, and Field Conditioning, PLoS One, 2019, 14(9), e0222719, DOI:10.1371/journal.pone.0222719.
- J. Xiong, S. Ren, Y. He, X. C. Wang, X. Bai, J. Wang and M. Dzakpasu, Bioretention Cell Incorporating Fe-Biochar and Saturated Zones for Enhanced Stormwater Runoff Treatment, Chemosphere, 2019, 237, 124424, DOI:10.1016/j.chemosphere.2019.124424.
- L. Zhao, H. Nan, Y. Kan, X. Xu, H. Qiu and X. Cao, Infiltration Behavior of Heavy Metals in Runoff through Soil Amended with Biochar as Bulking Agent, Environ. Pollut., 2019, 254, 113114, DOI:10.1016/j.envpol.2019.113114.
- A. B. Boehm, C. Bell, N. J. M. Fitzgerald, E. Gallo, C. P. Higgins, T. S. Hogue, R. G. Luthy, A. Portmann and B. A. Ulrich, Data Collected during Systematic Review of Biochar Effectiveness at Removing Stormwater Contaminants from Stormwater, Stanf. Digit. Repos., 2020, https://purl.stanford.edu/qs993pr7111 Search PubMed.
- Y. Tong, P. J. McNamara and B. K. Mayer, Adsorption of Organic Micropollutants onto Biochar: A Review of Relevant Kinetics, Mechanisms and Equilibrium, Environ. Sci.: Water Res. Technol., 2019, 5(5), 821–838, 10.1039/C8EW00938D.
- H. Li, X. Dong, E. B. da Silva, L. M. de Oliveira, Y. Chen and L. Q. Ma, Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications, Chemosphere, 2017, 178, 466–478, DOI:10.1016/j.chemosphere.2017.03.072.
- J. Tian, V. Miller, P. C. Chiu, J. A. Maresca, M. Guo and P. T. Imhoff, Nutrient Release and Ammonium Sorption by Poultry Litter and Wood Biochars in Stormwater Treatment, Sci. Total Environ., 2016, 553, 596–606, DOI:10.1016/j.scitotenv.2016.02.129.
- D. V. Sarkhot, T. A. Ghezzehei and A. A. Berhe, Effectiveness of Biochar for Sorption of Ammonium and Phosphate from Dairy Effluent, J. Environ. Qual., 2013, 42, 1545–1554, DOI:10.2134/jeq2012.0482.
- Y. Yao, B. Gao, M. Zhang, M. Inyang and A. R. Zimmerman, Effect of Biochar Amendment on Sorption and Leaching of Nitrate, Ammonium, and Phosphate in a Sandy Soil, Chemosphere, 2012, 89(11), 1467–1471, DOI:10.1016/j.chemosphere.2012.06.002.
- B. S. L. Coleman, Z. M. Easton and E. M. Bock, Biochar Fails to Enhance Nutrient Removal in Woodchip Bioreactor Columns Following Saturation, J. Environ. Manage., 2019, 232, 490–498, DOI:10.1016/j.jenvman.2018.11.074.
- B. Chen, D. Zhou and L. Zhu, Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures, Environ. Sci. Technol., 2008, 42(14), 5137–5143, DOI:10.1021/es8002684.
- D. Zhu, S. Kwon and J. J. Pignatello, Adsorption of Single-Ring Organic Compounds to Wood Charcoals Prepared under Different Thermochemical Conditions, Environ. Sci. Technol., 2005, 39(11), 3990–3998, DOI:10.1021/es050129e.
- G. Cornelissen, J. Haftka, J. Parsons and Ö. Gustafsson, Sorption to Black Carbon of Organic Compounds with Varying Polarity and Planarity, Environ. Sci. Technol., 2005, 39(10), 3688–3694, DOI:10.1021/es048346n.
- L. Klüpfel, M. Keiluweit, M. Kleber and M. Sander, Redox Properties of Plant Biomass-Derived Black Carbon (Biochar), Environ. Sci. Technol., 2014, 48(10), 5601–5611, DOI:10.1021/es500906d.
- J. M. Saquing, Y.-H. Yu and P. C. Chiu, Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor, Environ. Sci. Technol. Lett., 2016, 3(2), 62–66, DOI:10.1021/acs.estlett.5b00354.
- A. Kappler, M. L. Wuestner, A. Ruecker, J. Harter, M. Halama and S. Behrens, Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals, Environ. Sci. Technol. Lett., 2014, 1(8), 339–344, DOI:10.1021/ez5002209.
- J. Lehmann and S. Joseph, Biochar Production Technology, in Biochar for Environmental Management Science and Technology, 2012, pp. 127–139 Search PubMed.
- M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review, Chemosphere, 2014, 99, 19–33, DOI:10.1016/j.chemosphere.2013.10.071.
- S. M. Abit, C. H. Bolster, P. Cai and S. L. Walker, Influence of Feedstock and Pyrolysis Temperature of Biochar Amendments on Transport of Escherichia Coli in Saturated and Unsaturated Soil, Environ. Sci. Technol., 2012, 46(15), 8097–8105, DOI:10.1021/es300797z.
- S. Sasidharan, S. Torkzaban, S. A. Bradford, R. Kookana, D. Page and P. G. Cook, Transport and Retention of Bacteria and Viruses in Biochar-Amended Sand, Sci. Total Environ., 2016, 548–549, 100–109, DOI:10.1016/j.scitotenv.2015.12.126.
- D. Sedlak, Sifting Through the Embers, Environ. Sci. Technol., 2018, 52(6), 3327–3328, DOI:10.1021/acs.est.8b01200.
- Z. Mahdi, Q. J. Yu and A. El Hanandeh, Investigation of the Kinetics and Mechanisms of Nickel and Copper Ions Adsorption from Aqueous Solutions by Date Seed Derived Biochar, J. Environ. Chem. Eng., 2018, 6(1), 1171–1181, DOI:10.1016/j.jece.2018.01.021.
- D. Kołodyńska, R. Wnętrzak, J. J. Leahy, M. H. B. Hayes, W. Kwapiński and Z. Hubicki, Kinetic and Adsorptive Characterization of Biochar in Metal Ions Removal, Chem. Eng. J., 2012, 197, 295–305, DOI:10.1016/j.cej.2012.05.025.
- S. Kang, J. Jung, J. K. Choe, Y. S. Ok and Y. Choi, Effect of Biochar Particle Size on Hydrophobic Organic Compound Sorption Kinetics: Applicability of Using Representative Size, Sci. Total Environ., 2018, 619–620, 410–418, DOI:10.1016/j.scitotenv.2017.11.129.
- W. Zheng, M. Guo, T. Chow, D. N. Bennett and N. Rajagopalan, Sorption Properties of Greenwaste Biochar for Two Triazine Pesticides, J. Hazard. Mater., 2010, 181(1), 121–126, DOI:10.1016/j.jhazmat.2010.04.103.
- S. Abel, A. Peters, S. Trinks, H. Schonsky, M. Facklam and G. Wessolek, Impact of Biochar and Hydrochar Addition on Water Retention and Water Repellency of Sandy Soil, Geoderma, 2013, 202–203, 183–191, DOI:10.1016/j.geoderma.2013.03.003.
- J. Tian, J. Jin, P. C. Chiu, D. K. Cha, M. Guo and P. T. Imhoff, A Pilot-Scale, Bi-Layer Bioretention System with Biochar and Zero-Valent Iron for Enhanced Nitrate Removal from Stormwater, Water Res., 2019, 148, 378–387, DOI:10.1016/j.watres.2018.10.030.
- E. G. I. Payne, T. Pham, P. L. M. Cook, T. D. Fletcher, B. E. Hatt and A. Deletic, Biofilter Design for Effective Nitrogen Removal from Stormwater – Influence of Plant Species, Inflow Hydrology and Use of a Saturated Zone, Water Sci. Technol., 2014, 69(6), 1312–1319 CrossRef CAS PubMed.
- Z. Liu, B. Dugan, C. A. Masiello, R. T. Barnes, M. E. Gallagher and H. Gonnermann, Impacts of Biochar Concentration and Particle Size on Hydraulic Conductivity and DOC Leaching of Biochar–Sand Mixtures, J. Hydrol., 2016, 533, 461–472, DOI:10.1016/j.jhydrol.2015.12.007.
- M. O. Omondi, X. Xia, A. Nahayo, X. Liu, P. K. Korai and G. Pan, Quantification of Biochar Effects on Soil Hydrological Properties Using Meta-Analysis of Literature Data, Geoderma, 2016, 274, 28–34, DOI:10.1016/j.geoderma.2016.03.029.
- R. T. Barnes, M. E. Gallagher, C. A. Masiello, Z. Liu and B. Dugan, Biochar-Induced Changes in Soil Hydraulic Conductivity and Dissolved Nutrient Fluxes Constrained by Laboratory Experiments, PLoS One, 2014, 9(9), e108340, DOI:10.1371/journal.pone.0108340.
- P. de Rozari, M. Greenway and A. El Hanandeh, Nitrogen Removal from Sewage and Septage in Constructed Wetland Mesocosms Using Sand Media Amended with Biochar, Ecol. Eng., 2018, 111, 1–10, DOI:10.1016/j.ecoleng.2017.11.002.
- H. M. Ibrahim, M. I. Al-Wabel, A. R. A. Usman and A. Al-Omran, Effect of Conocarpus Biochar Application on the Hydraulic Properties of a Sandy Loam Soil, Soil Sci., 2013, 178(4), 165–173 CrossRef CAS.
- A. J. Jefferson, A. S. Bhaskar, K. G. Hopkins, R. Fanelli, P. M. Avellaneda and S. K. McMillan, Stormwater Management Network Effectiveness and Implications for Urban Watershed Function: A Critical Review, Hydrol. Processes, 2017, 1–25, DOI:10.1002/hyp.11347.
- H. E. Golden and N. Hoghooghi, Green Infrastructure and Its Catchment-Scale Effects: An Emerging Science, Wiley Interdiscip. Rev.: Water, 2018, 5(1), e1254, DOI:10.1002/wat2.1254.
- J. M. Wolfand, C. D. Bell, A. B. Boehm, T. S. Hogue and R. G. Luthy, Multiple Pathways to Bacterial Load Reduction by Stormwater Best Management Practices: Trade-Offs in Performance, Volume, and Treated Area, Environ. Sci. Technol., 2018, 52(11), 6370–6379, DOI:10.1021/acs.est.8b00408.
- J. M. Wolfand, C. Seller, C. D. Bell, Y.-M. Cho, K. Oetjen, T. S. Hogue and R. G. Luthy, Occurrence of Urban-Use Pesticides and Management with Enhanced Stormwater Control Measures at the Watershed Scale, Environ. Sci. Technol., 2019,(7), 3634–3644, DOI:10.1021/acs.est.8b05833.
- E. S. Bedan and J. C. Clausen, Stormwater Runoff Quality and Quantity from Traditional and Low Impact Development Watersheds, J. Am. Water Resour. Assoc., 2009, 45(4), 998–1008, DOI:10.1111/j.1752-1688.2009.00342.x.
- M. J. Burns, T. D. Fletcher, C. J. Walsh, S. J. Imberger, H. Duncan and C. Li Hydrologic and Water Quality Responses to Catchment-Wide Implementation of Stormwater Control Measures, in Novatech, 2016, pp. 1–4 Search PubMed.
- M. J. Pennino, R. I. McDonald and P. R. Jaffe, Watershed-Scale Impacts of Stormwater Green Infrastructure on Hydrology, Nutrient Fluxes, and Combined Sewer Overflows in the Mid-Atlantic Region, Sci. Total Environ., 2016, 565, 1044–1053, DOI:10.1016/j.scitotenv.2016.05.101.
- V. Gagrani, J. A. Diemer, J. J. Karl and C. J. Allan, Assessing the Hydrologic and Water Quality Benefits of a Network of Stormwater Control Measures in a SE U.S. Piedmont Watershed, J. Am. Water Resour. Assoc., 2014, 50(1), 128–142, DOI:10.1111/jawr.12121.
- E. Gallo, B. Colin, K. Mika, M. Gold and T. S. Hogue, Stormwater Management Options and Decision-Making in Urbanized Watersheds of Los Angeles, California, J. Sustain. Water Built Environ., 2020, 6(2), 04020003, DOI:10.1061/JSWBAY.0000905.
- E. Gallo, C. Bell, K. Mika, M. Gold and T. S. Hogue, Stormwater Management Options and Decision-Making in the Urbanized Watersheds of Los Angeles, CA, J. Sustain. Water Built Environ., 2020, 6(2), 04020003, DOI:10.1061/JSWBAY.0000905.
- K. Mika, E. Gallo, E. Porse, T. S. Hogue, S. Pincetl and M. GoldLA Sustainable Water Project: Los Angeles City-Wide Overview, 2018 Search PubMed.
- J. Gan, S. Bondarenko, L. Oki, D. Haver and J. X. Li, Occurrence of Fipronil and Its Biologically Active Derivatives in Urban Residential Runoff, Environ. Sci. Technol., 2012, 46(3), 1489–1495, DOI:10.1021/es202904x.
- D. P. Weston, R. W. Holmes and M. J. Lydy, Residential Runoff as a Source of Pyrethroid Pesticides to Urban Creeks, Environ. Pollut., 2009, 157(1), 287–294, DOI:10.1016/j.envpol.2008.06.037.
- D. P. Weston, D. Chen and M. J. Lydy, Stormwater-Related Transport of the Insecticides Bifenthrin, Fipronil, Imidacloprid, and Chlorpyrifos into a Tidal Wetland, San Francisco Bay, California, Sci. Total Environ., 2015, 527–528, 18–25, DOI:10.1016/j.scitotenv.2015.04.095.
- W. Zhang, J. Zheng, P. Zheng and R. Qiu, Atrazine Immobilization on Sludge Derived Biochar and the Interactive Influence of Coexisting Pb(II) or Cr(VI) Ions, Chemosphere, 2015, 134, 438–445, DOI:10.1016/j.chemosphere.2015.05.011.
- J. H. Park, J. S. Cho, Y. S. Ok, S. H. Kim, S. W. Kang, I. W. Choi, J. S. Heo, R. D. Delaune and D. C. Seo, Competitive Adsorption and Selectivity Sequence of Heavy Metals by Chicken Bone-Derived Biochar: Batch and Column Experiment, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2015, 50(11), 1194–1204, DOI:10.1080/10934529.2015.1047680.
- L. A. Rossman, Modeling Low Impact Development Alternatives with SWMM, J. Water Manag. Model., 2010, R236-11, DOI:10.14796/JWMM.R236-11.
- M. Leisenring, M. Barrett, C. Pomeroy, A. Poresky, L. Larry Roesner, A. C. Rowney and E. StreckerLinking BMP Systems Performance to Receiving Water Protection: BMP Performance Algorithms, SWC1R06bmp, 2013 Search PubMed.
- Y. Liu, B. A. Engel, D. C. Flanagan, M. W. Gitau, S. K. McMillan, I. Chaubey and S. Singh, Modeling Framework for Representing Long-Term Effectiveness of Best Management Practices in Addressing Hydrology and Water Quality Problems: Framework Development and Demonstration Using a Bayesian Method, J. Hydrol., 2018, 560, 530–545, DOI:10.1016/j.jhydrol.2018.03.053.
- K. Kasak, J. Truu, I. Ostonen, J. Sarjas, K. Oopkaup, P. Paiste, M. Kõiv-Vainik, Ü. Mander and M. Truu, Biochar Enhances Plant Growth and Nutrient Removal in Horizontal Subsurface Flow Constructed Wetlands, Sci. Total Environ., 2018, 639, 67–74, DOI:10.1016/j.scitotenv.2018.05.146.
- C. I. Kammann, S. Linsel, J. W. Gößling and H. W. Koyro, Influence of Biochar on Drought Tolerance of Chenopodium Quinoa Willd and on Soil-Plant Relations, Plant Soil, 2011, 345(1), 195–210, DOI:10.1007/s11104-011-0771-5.
- V. Venkataramanan, A. I. Packman, D. R. Peters, D. Lopez, D. J. McCuskey, R. I. McDonald, W. M. Miller and S. L. Young, A Systematic Review of the Human Health and Social Well-Being Outcomes of Green Infrastructure for Stormwater and Flood Management, J. Environ. Manage., 2019, 246, 868–880, DOI:10.1016/j.jenvman.2019.05.028.
- M. Demuzere, K. Orru, O. Heidrich, E. Olazabal, D. Geneletti, H. Orru, A. G. Bhave, N. Mittal, E. Feliu and M. Faehnle, Mitigating and Adapting to Climate Change: Multi-Functional and Multi-Scale Assessment of Green Urban Infrastructure, J. Environ. Manage., 2014, 146, 107–115, DOI:10.1016/j.jenvman.2014.07.025.
- D. J. Nowak, E. J. Greenfield, R. E. Hoehn and E. Lapoint, Carbon Storage and Sequestration by Trees in Urban and Community Areas of the United States, Environ. Pollut., 2013, 178, 229–236, DOI:10.1016/j.envpol.2013.03.019.
- D. J. Nowak, S. Hirabayashi, A. Bodine and R. Hoehn, Modeled PM2.5 Removal by Trees in Ten U.S. Cities and Associated Health Effects, Environ. Pollut., 2013, 178, 395–402, DOI:10.1016/j.envpol.2013.03.050.
- R. W. F. Cameron, T. Blanuša, J. E. Taylor, A. Salisbury, A. J. Halstead, B. Henricot and K. Thompson, The Domestic Garden - Its Contribution to Urban Green Infrastructure, Urban For. Urban Green., 2012, 11(2), 129–137, DOI:10.1016/j.ufug.2012.01.002.
- M. Mansor, I. Said and I. Mohamad, Experiential Contacts with Green Infrastructure's Diversity and Well-Being of Urban Community, Procedia Soc. Behav. Sci., 2012, 49, 257–267, DOI:10.21834/aje-bs.v2i2.178.
- K. Spahr, C. Bell, J. McCray and T. Hogue, Greening up Stormwater Infrastructure: Measuring Vegetation to Establish Context and Promote Co-Benefits in a Diverse Set of US Cities, Urban For. Urban Green., 2012, 11(2), 129–137, DOI:10.1016/j.ufug.2019.126548.
- H. A. Alhashimi and C. B. Aktas, Life Cycle Environmental and Economic Performance of Biochar Compared with Activated Carbon: A Meta-Analysis, Resour., Conserv. Recycl., 2017, 118, 13–26, DOI:10.1016/j.resconrec.2016.11.016.
- S. Shackley, A. Clare, S. Joseph, B. A. McCarl and H.-P. Schmidt Economic Evaluation of Biochar Systems, in Biochar for Environmental Management, Routledge, 2015, pp. 813–851 Search PubMed.
- USGS, Historical Statistics for Mineral and Material Commodities in the United States https://www.usgs.gov/centers/nmic/historical-statistics-mineral-and-material-commodities-united-states#sandandgravelcon (accessed Dec 1, 2019).
- K. G. Roberts, B. A. Gloy, S. Joseph, N. R. Scott and J. Lehmann, Life Cycle Assessment of Biochar Systems: Estimating the Energetic, Economic, and Climate Change Potential, Environ. Sci. Technol., 2010, 44(2), 827–833, DOI:10.1021/es902266r.
- Potomac Economics, Annual Report on the Market for RGGI CO2 Allowances: 2018, April, RGGI, Inc., 2019 Search PubMed.
- A. P. Davis, M. Shokouhian, H. Sharma, C. Minami and D. Winogradoff, Water Quality Improvement through Bioretention: Lead, Copper, and Zinc Removal, Water Environ. Res., 2003, 75(1), 73–82, DOI:10.2175/106143003X140854.
- N. Ashoori, M. Teixido, S. Spahr, G. H. LeFevre, D. L. Sedlak and R. G. Luthy, Evaluation of Pilot-Scale Biochar-Amended Woodchip Bioreactors to Remove Nitrate, Metals, and Trace Organic Contaminants from Urban Stormwater Runoff, Water Res., 2019, 154, 1–11, DOI:10.1016/j.watres.2019.01.040.
- S. Le Coustumer, T. D. Fletcher, A. Deletic, S. Barraud and P. Poelsma, The Influence of Design Parameters on Clogging of Stormwater Biofilters: A Large-Scale Column Study, Water Res., 2012, 46(20), 6743–6752, DOI:10.1016/j.watres.2012.01.026.
- C. Hsieh and A. Davis, Evaluation and Optimization of Bioretention Media for Treatment of Urban Storm Water Runoff, J. Environ. Eng., 2005, 131(11), 1521–1531, DOI:10.1061/(ASCE)0733-9372(2005)131:11(1521).
- J. Clary, B. Urbonas, J. Jones, E. Strecker, M. Quigley and J. O'Brien, Developing, Evaluating and Maintaining a Standardized Stormwater BMP Effectiveness Database, Water Sci. Technol., 2002, 45(7), 65–73 CrossRef CAS PubMed.
- J. Clary, M. Quigley, J. Jones and E. Strecker, International Stormwater BMP Database Enhancements and Updated Findings, Proc. Water Environ. Fed., 2007, vol. 2007, (13), pp. 5603–5616 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew00027b |
| This journal is © The Royal Society of Chemistry 2020 |
