Hemolytic and cellular toxicology of a sulfanilamide-based nonionic surfactant: a niosomal carrier for hydrophobic drugs†
Abstract
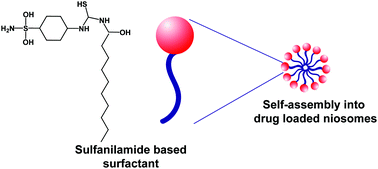
Biocompatible surfactants are of diverse pharmaceutical interest due to their ability to self-assemble into nano-particulate systems which can be used for single-step drug loading, based upon the hydrophobic–hydrophobic interaction between a hydrophobic drug and the lipophilic part of a surfactant molecule. However, surfactants are associated with cytotoxicity and hemolysis due to their amphiphilic interaction with cellular membranes. This study reports a novel membrane-compatible surfactant, synthesized from sulfanilamide and its self-micellization into niosomes. The surfactant was synthesized in a single step reaction via the introduction of an alkyl chain in the sulfanilamide moiety by conjugation with deconyl chloride. The synthesized surfactant (S-SDC) was characterized by 1H and 13C NMR, mass spectrometry and single crystal XRD. The S-SDC niosomes were explored for drug delivery with clarithromycin as a model drug. The biocompatibility of the surfactant was investigated through hemolysis and cytotoxicity. The surfactant presented a very low critical micellar concentration (CMC) of 0.04 mM and entrapped 65% of the drug which was released in a sustained manner, over 12 h, at acidic and physiological pH. The vesicles were spherical in shape with 234 ± 3.61 nm mean diameter and a narrow size distribution. Niosomes were hemocompatible and nontoxic to cellular membrane. The results suggested the sulfanilamide based surfactant can be applied as a novel and cell membrane compatible niosomal drug delivery vehicle.
- This article is part of the themed collection: Toxicology Research Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...