Are metals and pyrene levels additional factors playing a pivotal role in air pollution-induced inflammation in taxi drivers?
Bruna
Gauer
ab,
Natália
Brucker
c,
Anelise
Barth
a,
Marcelo D.
Arbo
ab,
Adriana
Gioda
d,
Flávia V.
Thiesen
e,
Jessica
Nardi
ab and
Solange C.
Garcia
 *ab
*ab
aLaboratory of Toxicology (LATOX), Department of Analysis, Universidade Federal do Rio Grande do Sul, Av. Ipiranga 2752, 90610-000, Porto Alegre, RS, Brazil. E-mail: solange.garcia@ufrgs.br; Fax: (+55) 51 3308-5437; Tel: (+55)51 3308-5297
bPost-Graduate Program in Pharmaceutical Sciences, Universidade Federal do Rio Grande do Sul, Av. Ipiranga 2752, 90610-000, Porto Alegre, RS, Brazil
cDepartment of Physiology and Pharmacology, Federal University of Santa Maria, Roraima 1000, 97105-900, Santa Maria, RS, Brazil
dDepartment of Chemistry, Pontifical Catholic University of Rio de Janeiro (PUC-Rio), Rua Marquês de São Vicente 225, 22451-900, Rio de Janeiro, RJ, Brazil
ePharmacy Faculty and Toxicology Institute, Pontifical Catholic University of Rio Grande do Sul, Av. Ipiranga 6681, 90619-900, Porto Alegre, RS, Brazil
First published on 10th October 2017
Abstract
This study aimed to evaluate which xenobiotic (As, Hg, Pb or pyrenes) is primarily responsible for the inflammatory process in taxi drivers. Multiple regression analysis showed that Hg is the main xenobiotic responsible for the increase of cytokine levels. These associations suggest that co-exposure to pollutants could be a risk factor for health effects.
Introduction
Traffic-derived emissions are a prominent source of urban pollution, due to their ubiquity and increasing prevalence, especially in urban areas.1 The World Health Organization estimated that 23% of all deaths that occurred in 2012 were attributable to environmental factors and that around 7 million people died – one in eight of total global deaths – as a result of air pollution exposure.2,3 Environmental pollution exposure has been associated with a risk factor in the morbidity and mortality of respiratory and cardiovascular diseases.4,5Motor vehicles emit a large mixture of several harmful chemical components, such as toxic gases, particulate matter (PM) and other residues of incomplete combustion,6 into the atmosphere. The PM released into the environment carries various constituents that can cause damaging health effects adsorbed onto its surface, including polycyclic aromatic hydrocarbons (PAHs) and heavy metals.7,8 Traffic-related PM is the major source of air pollution in metropolitan areas, and outdoor workers such as taxi drivers are expected to have a greater exposure to air pollutants than the general population.9 1-Hydroxypyrene (1-OHP) is a metabolite of pyrenes, and it is considered the main biomarker for assessing exposure to PAHs, since pyrenes are present in high concentrations in mixtures of PAHs. There is a good correlation between external exposure from traffic-related air pollution and excretion levels of 1-OHP in urine.10
It is established that pyrenes associated with air PM lead to reactive oxygen species (ROS) production in the organism, resulting in oxidative and inflammatory responses.11 Likewise, it has been observed that the presence of PAHs jointly with metals in traffic exhaust particles has substantial pro-inflammatory and oxidative effects.12
Studies performed in our laboratory have already demonstrated that the occupational exposure of taxi drivers to PAHs and metals present in traffic-air pollution correlated with oxidative and inflammatory damage.13,14 It is known that atmospheric pollution is due to a complex mixture of chemical agents and in the present work some agents were evaluated. On the other hand, we concentrated on analysing the main metabolite of pyrenes, 1-OHP, and trace metals, e.g. As, Hg and Pb, because these pollutants showed the most pro-oxidant and inflammatory effects, according to our previous results from studies with taxi drivers.15 Although these previous studies have shown an association between air pollution and inflammation, few studies have examined additive or synergistic effects. This study focused on evaluating which of these xenobiotics (metals or pyrenes) is primarily responsible for the inflammatory process observed in taxi drivers. Therefore, we have gathered data from individuals recruited over three years of study in order to better evaluate these risks of exposure.
Materials and methods
This study comprised one hundred and thirty two non-smoker males. Subjects older than 60 years, smokers, with a history of chronic diseases, taking vitamin supplementation and those who had failed to collect samples or participate in any stage of the study were excluded. The group of workers who are potentially exposed to outdoor air pollution on the streets during their daily work consisted of 80 taxi drivers of Porto Alegre, the capital of Rio Grande do Sul (RS), a southern state in Brazil. The city is located in the extreme south of the country, with approximately 1.5 million habitants and 822 thousand licensed vehicles in circulation, resulting in a mean of 1.8 inhabitants per vehicle (Detran/2016). The group of workers who are expected not to be exposed to outdoor pollution at work consisted of 52 individuals with administrative occupation and living in the same city as the exposed group. The mean age of taxi drivers was higher (47.78 ± 1.11 years) than that of the control group (40.87 ± 1.48 years) (p < 0.01).The participants were recruited through advertising and leafleting. Both the groups were simultaneously submitted to equivalent examinations and procedures. The Committee on Research Ethics at Federal University of Rio Grande do Sul (No. 20322/11) approved this study. All the participants were informed about the study and signed a consent form according to the guidelines of the local committee.
All recruitment and sample collections were performed during winter because this season is characterized by high levels of air pollutants. Pre-work shift urine was collected for the determination of 1-hydroxypyrene and creatinine levels. Blood samples were collected from all participants by venipuncture into Vacutainer™ tubes. Blood–heparin tubes were collected and aliquots were stored at −20 °C inside free metal tubes until analysis to determine the toxic metallic elements lead (Pb), mercury (Hg) and arsenic (As). A tube, collected without anticoagulant, was centrifuged at 1500g for 10 min at room temperature and the serum obtained was frozen and kept under −80 °C for subsequent determination of inflammatory cytokines.
The levels of the urinary metabolite of pyrenes, 1-hydroxypyrene (1-OHP), were determined in urine samples by the enzymatic hydrolysis of the conjugated metabolite, followed by solid phase extraction and analysis of the reconstituted extract by high performance liquid chromatography (HPLC) equipped with a fluorescence detector.14 Urinary creatinine was determined by spectrophotometry using commercial kits (Doles, Brazil). The concentrations of 1-OHP were expressed in μmol mol−1 creatinine.
The levels of metals mercury (Hg) and lead (Pb) and the metalloid arsenic (As) in whole blood were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS; PerkinElmer-Sciex).15 As and Hg were expressed as μg l−1, while Pb as μg dl−1.
Inflammatory cytokines were quantified using immunologic ELISA methods for human interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) (R&D Systems), according to the manufacturer's instructions. The results were expressed as pg ml−1.
Statistical analysis was performed using the SPSS software (version 22). Normality was assessed through the Shapiro–Wilk test. Results were reported as mean ± standard error of the mean (SEM). Comparisons between groups were achieved by ANCOVA covariation adjusted for age. Multiple linear regression analyses were performed to evaluate whether inflammatory biomarkers are influenced by trace elements and 1-OHP, using as covariates age, 1-OHP, As, Hg and Pb. Values of p ≤ 0.05 were considered significant.
Results and discussion
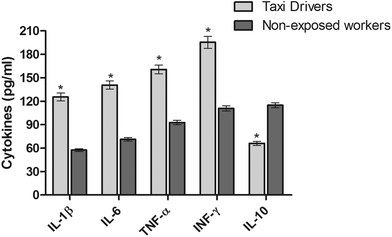
Experimental and epidemiological studies have highlighted the association of PM with negative respiratory and cardiovascular effects, the inflammatory process being an important mechanism behind these diseases.16,17 Furthermore, it is already proved that PM is able to carry various compounds like metals and pyrenes adsorbed onto its surface, and they, in turn, are responsible for triggering the inflammation that can cause tissue damage.18,19The results obtained in the present study showed that the concentrations of toxic elements (As, Hg, and Pb) quantified in whole blood were significantly higher in taxi drivers in relation to the non-exposed group, controlled by age (p < 0.001) (Table 1). The same was observed in the urinary levels of 1-OHP (Table 1). Regarding the serum biomarkers of inflammation, significant increases of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α and IFN-γ) were observed in the taxi drivers, while the anti-inflammatory cytokine IL-10 was lower in this group compared with the control (p < 0.001) (Fig. 1).
| Taxi drivers (n = 80) | Non-exposed (n = 52) | Limits recommended20 | |
|---|---|---|---|
| Abbreviations: As: arsenic. Hg: mercury. Pb: lead. 1-OHP: 1-hydroxypyrene. Results are expressed as mean ± SEM. ANCOVA covariation, adjusted for age: *p < 0.01 compared with non-exposed individuals. **p < 0.001 compared with non-exposed individuals. | |||
| As (μg l−1) | 19.56 ± 0.61** | 13.18 ± 0.50 | 2 to 20 |
| Hg (μg l−1) | 17.79 ± 2.43** | 2.75 ± 0.32 | 2 to 20 |
| Pb (μg dl−1) | 2.08 ± 0.15** | 1.29 ± 0.07 | 5 to 15 |
| 1-OHP (μmol mol−1 creatinine) | 0.114 ± 0.010* | 0.077 ± 0.004 | — |
There is particular concern that co-exposure to PAHs (pyrenes) and toxic elements may result in additive or synergistic effects. A multiple regression analysis was performed to evaluate which of these risk factors may influence more on the inflammatory biomarkers (Table 2). Interestingly, blood Hg was the major predictor of increase in all pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-γ) and decrease in the anti-inflammatory cytokine IL-10 levels. This model accounted for 33% IL-1β (R2 = 0.329), 37% IL-6 (R2 = 0.371), 36% TNF-α (R2 = 0.357), 43% IFN-γ (R2 = 0.425) and for 25% IL-10 (R2 = 0.252). It was demonstrated that other chemical agents contribute to the inflammatory process, but the role of Hg in environment-exposed workers must not be underestimated. Nevertheless, the urinary levels of 1-OHP were the second most important biomarker associated with an increase of the cytokines IL-1β, IL-6, TNF-α and IFN-γ, while the arsenic levels in blood were the second best marker of decreased IL-10. The parameters age and Pb levels in the blood were weak predictors of IFN-γ changes in this model.
| IL-1β (pg ml−1) | IL-6 (pg ml−1) | TNF-α (pg ml−1) | IFN-γ (pg ml−1) | IL-10 (pg ml−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R 2 = 0.329 | R 2 = 0.371 | R 2 = 0.357 | R 2 = 0.425 | R 2 = 0.252 | ||||||
| β | p-Values | β | p-Values | β | p-Values | β | p-Values | β | p-Values | |
| As: arsenic. Hg: mercury. Pb: lead. 1-OHP: 1-hydroxypyrene. IL-1β: interleukin-1β. IL-6: interleukin-6. TNF-α: tumor necrosis factor-α. IFN-γ: interferon-γ. IL-10: interleukin-10. | ||||||||||
| Age (years) | 0.033 | 0.686 | −0.063 | 0.425 | −0.130 | 0.104 | −0.155 | 0.040 | 0.010 | 0.908 |
| As (μg L−1) | 0.199 | 0.016 | 0.211 | 0.008 | 0.184 | 0.022 | 0.152 | 0.046 | −0.228 | 0.009 |
| Hg (μg L−1) | 0.435 | < 0.001 | 0.447 | < 0.001 | 0.409 | < 0.001 | 0.527 | < 0.001 | −0.388 | < 0.001 |
| Pb (μg dL−1) | 0.094 | 0.300 | 0.173 | 0.051 | 0.234 | 0.009 | 0.214 | 0.012 | −0.033 | 0.732 |
| 1-OHP (μmol mol−1 creatinine) | 0.244 | 0.003 | 0.267 | 0.001 | 0.292 | < 0.001 | 0.214 | 0.005 | −0.222 | 0.011 |
The taxi drivers presented a mean concentration next to the maximum limit of As and Hg, while the levels of Pb were within the reference value.20 Although the concentrations found in taxi drivers do not exceed the maximum value established by WHO, it was possible to observe significant changes in the inflammatory markers in the exposed group. In this context, our results indicated that relatively normal levels of exposure to these toxic elements are potentially harmful to these workers. Furthermore, applying biomarkers of exposure and effect could be useful in providing insight into the biological mechanisms.
In this study, blood Hg was the major marker influencing the increase of pro-inflammatory cytokines. This is in accordance with our previous results; however, the previous statistic model used did not consider 1-OHP as a parameter. The deleterious effects induced by Hg are already described, since this metal accumulates in several tissues.21 Data from in vitro and in vivo studies indicate the involvement of Hg in the development of the inflammatory process by ROS production due to its physicochemical properties.22,23 However, human exposure effects depend on the chemical form and sources of exposure that include the inhalation of polluted air or the ingestion of contaminated food.24 In fact, exposure to multiple chemicals may lead to many interactions with a wide array of underlying mechanisms that may result in diverse health outcomes. Air pollution exposure contributes to an increase in the inflammatory process; however, other factors may also influence inflammation, such as the time of exposure, lipid profile, and genetic chronic diseases.25,26 In this line, the weak correlation coefficients found could be justified by the mixture of chemical pollutant agents in air, which may be affecting the individual correlations obtained for exposure biomarkers when they are grouped in the multifactorial analysis.
Although 1-OHP was not the main predictor of the changes in inflammatory biomarkers by our statistical model, we cannot underestimate its contribution to the inflammatory process triggered by occupational exposure to air pollution. PAHs are able to stimulate inflammation through different ways and mechanisms, most of them are related to oxidative stress. An example is the aryl hydrocarbon receptor (AhR) pathway, where PAHs bind to the receptor and then activate the expression of genes such as CYP1A1, which is related to the excessive production of ROS and the formation of DNA adducts. The activation of AhR also stimulates the production of COX-2, the first step in the inflammatory cascade.27
Conclusions
The results revealed that co-exposure to xenobiotics related to the traffic air pollution such as Hg, As, Pb, and pyrenes can significantly contribute to the inflammatory process in daily exposed workers. However, there are many other chemical agents in atmospheric pollution that also influence the inflammatory process, as suggested through a multiple linear regression model. This study has provided important evidence that Hg is the main xenobiotic responsible for the increase in the pro-inflammatory cytokines, as well as the decrease of the anti-inflammatory capacity represented by IL-10. This indicates that the detection of biomarkers is an important step to demonstrate health effects after exposure to pollutants. Besides this, the inflammation process has been proposed as the factor involved in mediating the pollutant effects on cardiovascular disease and this co-exposure to xenobiotics could be a risk factor for adverse health effects.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the FAPERGS and PPSUS grant to SC Garcia, CNPq, Capes. SC Garcia is a recipient of the CNPq research fellowship.References
- B. Niemann, S. Rohrbach, M. R. Miller, D. E. Newby, V. Fuster and J. C. Kovacic, Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series, J. Am. Coll. Cardiol., 2017, 70, 230–251 CrossRef CAS PubMed.
- P. Braveman and L. Gottlieb, The social determinants of health: it's time to consider the causes of the causes, Public Health Rep., 2014, 129, 19–31 CrossRef PubMed.
- P. Venkatesan, WHO report: air pollution is a major threat to health, Lancet Respir. Med., 2016, 4, 351 CrossRef PubMed.
- G. Hoek, R. M. Krishnan, R. Beelen, A. Peters, B. Ostro, B. Brunekreef and J. D. Kaufman, Long-term air pollution exposure and cardio- respiratory mortality: a review, Environ. Health, 2013, 12, 43 CrossRef CAS PubMed.
- B. Brunekreef and B. Hoffmann, Air pollution and heart disease, Lancet, 2016, 388, 640–642 CrossRef.
- F. J. Kelly and J. C. Fussell, Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter, Atmos. Environ., 2012, 60, 504–526 CrossRef CAS.
- T. Wang, W. Feng, D. Kuang, Q. Deng, W. Zhang, S. Wang, M. He, X. Zhang, T. Wu and H. Guo, The effects of heavy metals and their interactions with polycyclic aromatic hydrocarbons on the oxidative stress among coke-oven workers, Environ. Res., 2015, 140, 405–413 CrossRef CAS PubMed.
- K. Ravindra, R. Sokhi and R. Van Grieken, Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation, Atmos. Environ., 2008, 42, 2895–2921 CrossRef CAS.
- H. Choudhary and S. M. Tarlo, Airway effects of traffic-related air pollution on outdoor workers, Curr. Opin. Allergy Clin. Immunol., 2014, 14, 106–112 CrossRef CAS PubMed.
- M. Ciarrocca, M. V. Rosati, F. Tomei, A. Capozzella, G. Andreozzi, G. Tomei, A. Bacaloni, T. Casale, J. C. Andrè and M. Fioravanti, Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis, J. Exposure Anal. Environ. Epidemiol., 2014, 24, 17–26 CrossRef CAS PubMed.
- Y. Zhang, S. Dong, H. Wang, S. Tao and R. Kiyama, Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors, Environ. Pollut., 2016, 213, 809–824 CrossRef CAS PubMed.
- A. I. Totlandsdal, M. Låg, E. Lilleaas, F. Cassee and P. Schwarze, Differential proinflammatory responses induced by diesel exhaust particles with contrasting PAH and metal content, Environ. Toxicol., 2015, 30, 188–196 CrossRef CAS PubMed.
- A. Barth, N. Brucker, A. M. Moro, S. Nascimento, G. Goethel, C. Souto, R. Fracasso, E. Sauer, L. Altknecht and B. da Costa, Association between inflammation processes, DNA damage, and exposure to environmental pollutants, Environ. Sci. Pollut. Res., 2017, 24, 353–362 CrossRef CAS PubMed.
- N. Brucker, A. M. Moro, M. F. Charão, J. Durgante, F. Freitas, M. Baierle, S. Nascimento, B. Gauer, R. P. Bulcão and G. B. Bubols, Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers, Sci. Total Environ., 2013, 463, 884–893 CrossRef PubMed.
- N. Brucker, A. Moro, M. Charão, G. Bubols, S. Nascimento, G. Goethel, A. Barth, A. C. Prohmann, R. Rocha and R. Moresco, Relationship between blood metals and inflammation in taxi drivers, Clin. Chim. Acta, 2015, 444, 176–181 CrossRef CAS PubMed.
- A. Baulig, S. Singh, A. Marchand, R. Schins, R. Barouki, M. Garlatti, F. Marano and A. Baeza-Squiban, Role of Paris PM 2.5 components in the pro-inflammatory response induced in airway epithelial cells, Toxicology, 2009, 261, 126–135 CrossRef CAS PubMed.
- L. D. Knibbs and L. Morawska, Traffic-related fine and ultrafine particle exposures of professional drivers and illness: An opportunity to better link exposure science and epidemiology to address an occupational hazard?, Environ. Int., 2012, 49, 110–114 CrossRef CAS PubMed.
- J. M. Brito, L. Belotti, A. C. Toledo, L. Antonangelo, F. S. Silva, D. S. Alvim, P. A. Andre, P. H. Saldiva and D. H. Rivero, Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles, Toxicol. Sci., 2010, 116, 67–78 CrossRef CAS PubMed.
- N. L. Mills, K. Donaldson, P. W. Hadoke, N. A. Boon, W. MacNee, F. R. Cassee, T. Sandström, A. Blomberg and D. E. Newby, Adverse cardiovascular effects of air pollution, Nat. Clin. Pract. Cardiovasc. Med., 2009, 6, 36–44 CrossRef CAS PubMed.
- WHO, Trace elements in human nutrition and health, World Health Organization, 1996 Search PubMed.
- J. Aazami, A. Esmaili-Saria, N. Bahramifar and M. Savabieasfahani, Total and organic mercury in liver, kidney and muscle of waterbirds from wetlands of the Caspian Sea, Iran, Bull. Environ. Contam. Toxicol., 2012, 89, 96–101 CrossRef CAS PubMed.
- S. C. Bondy, in Inflammation, Aging, and Oxidative Stress, Springer, 2016, pp. 3–16 Search PubMed.
- S. H. Kim and R. P. Sharma, Cytotoxicity of inorganic mercury in murine T and B lymphoma cell lines: involvement of reactive oxygen species, Ca 2+ homeostasis, and cytokine gene expression, Toxicol. in Vitro, 2003, 17, 385–395 CrossRef CAS PubMed.
- A. A. Tinkov, O. P. Ajsuvakova, M. G. Skalnaya, E. V. Popova, A. I. Sinitskii, O. N. Nemereshina, E. R. Gatiatulina, A. A. Nikonorov and A. V. Skalny, Mercury and metabolic syndrome: a review of experimental and clinical observations, BioMetals, 2015, 28, 231–254 CrossRef CAS PubMed.
- N. Brucker, M. F. Charão, A. M. Moro, P. Ferrari, G. Bubols, E. Sauer, R. Fracasso, J. Durgante, F. V. Thiesen and M. M. Duarte, Atherosclerotic process in taxi drivers occupationally exposed to air pollution and co-morbidities, Environ. Res., 2014, 131, 31–38 CrossRef CAS PubMed.
- G. Pawelec, D. Goldeck and E. Derhovanessian, Inflammation, ageing and chronic disease, Curr. Opin. Immunol., 2014, 29, 23–28 CrossRef CAS PubMed.
- L. Yang, X.-Y. Hou, Y. Wei, P. Thai and F. Chai, Biomarkers of the health outcomes associated with ambient particulate matter exposure, Sci. Total Environ., 2017, 579, 1446–1459 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |