From C60Ph5Cl to C60Ph6: complete phenylation of C60 derivative renders superior organic photovoltaic performance†
Abstract
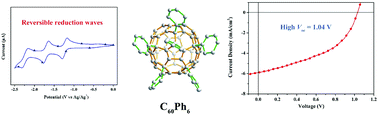
Fully phenylated C60Ph6 has never been synthesized in the past two decades largely due to steric effect and poor thermodynamic stability of the initial intermediate towards its isostructural penta-phenyl adduct (C60Ph5X) that has one central site intact. Herein, we report a kinetic management strategy using nitrobenzene that can efficiently alleviate the steric effect in the substitution reaction of C60Cl6 to synthesize C60Ph6. As revealed by X-ray crystallography, the molecule of C60Ph6, in stark contrast to its isostructural C60Ph5Cl with the last chlorine intact, shows a favorable geometrical structure and molecular stacking for electron transport. Furthermore, C60Ph6 shows high LUMO energy of −3.51 eV (0.4 eV higher than that of [6,6]-phenyl-C61-butyric acid methyl ester, PCBM), which is promising high open-circuit voltage for organic solar cells. The merits of both molecular packing and electrochemical property of C60Ph6 lead to high open-circuit voltage of 1.04 V for the OSC device based on poly(3-hexylthiophene) (P3HT). The present C60Ph6 exemplifies a novel class of electronic acceptors with fully aromatized groups on a fullerene cage.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...