Deciphering the potentiometric properties of (porphinato)zinc(ii)-derived supramolecular polymers and related superstructures†‡
Abstract
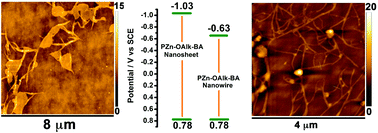
Because modulating the structure–function relationships of π-conjugated superstructures opens fresh opportunities to tune the electronic structures of semiconducting materials, self-assembled architectures have emerged as pivotal candidates to engineer optoelectronic devices. While the photophysical and electrical properties of 1-dimensional supramolecular polymers have been extensively explored, establishing their fundamental potentiometric properties using reliable electrochemical measurements has been less scrutinized and would benefit the engineering of semiconducting materials. In this regard, elucidating the energy level of valence and conduction bands that delineate the electronic structure of self-assemblies is critical to unveiling the parameters that regulate their structure–function properties. In the present contribution, design principles to engineer 2-dimensional nanosheets, nanowires, fibers and amorphous solids from (porphinato)zinc(II) (PZn) building blocks have been elucidated by modifying the structural properties of the side chains that flank PZn-based cores. As these self-assemblies feature identical redox-active building blocks but evidence different solid-state morphologies, the elucidation of their potentiometric properties reveals important structural parameters that regulate the potentials at which holes and electrons are injected into the valence and conduction bands of these hierarchical materials. While self-assembly conformations modestly impact valence band energies, superstructures built from H-type aggregates feature a conduction band energy stabilized by more than 350 meV with respect to those constructed from J-type aggregates.
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...