Efficient emissive fluorene-based p–n conjugated porous materials for near-white electroluminescence: benefits of metal-free Friedel–Crafts green polymerization†
Abstract
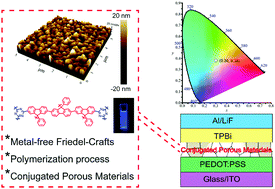
Efficient emissive fluorene-based p–n conjugated porous materials (CPMs) have been constructed via an effective Friedel–Crafts green polymerization. For metal-free and room-temperature processing, CPMs exhibit high fluorescence quantum efficiency of 32%, allowing the fabrication of near-white polymer light-emitting diodes with CIE of (0.30, 0.38).
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...